Invasion History
First Galapagos Record: 1989General Invasion History:
Bankia carinata was described from Sumatra in 1827 (Turner 1966), but its native range is unknown. It was already established in the region of Naples, Italy in 1829, and in the English Channel in 1875, and in India, Japan, and the Philippines (Turner 1966; Tsunoda 1979). An Indo-Pacific origin is likely, but it was apparently widely distributed around the world on shipping and drifting timber, by the 19th century (Turner 1966). In European waters, it occurs only in the Mediterranean Sea, at a temperature range of 9-30 C (Borges et al. 2014). It was found off naval ports in the Gulf of Mexico, Florida in 1922, at a lightship off North Carolina in 1951, and in drifting wood on Nantucket in 1949, and in the Gulf Stream south of Woods Hole in 1983 (Museum of Comparative Ecology records 2019).
Bankia carinata is widely distributed in tropical and subtropical waters around the world but appears to be rare in the East Pacific. It was found in Puntarenas, on the Gulf of Nicoya, Costa Rica in 1979, (Malacology 351683, Museum of Comparative Zoology 2019), and in Posorja and Guayas, Ecuador (Coan and Valentich-Scott 2012, cited by Treneman et al. 2018). This shipworm has been reported on a species list for San Diego (Coan and Scott 1997), but with no information on the date, establishment, or circumstances of collection.
Invasion History in the Galapagos:
Bankia carinata was first reported in the Galapagos Islands, Ecuador in 1989, in mangroves on the island of Baltra and was treated as rare by Cruz (1996).
Invasion history elsewhere in the world:
Bankia 'carinata' is likely a complex of several species, with populations in the Mediterranean, East Pacific and Caribbean showing genetic divergence. This species is likely to have been widely transported with wooden ships. Its larvae are planktonic (Turner and Johnson 1971; MacIntosh et al. 2014), so ballast water transport is also possible (Borges 2012).
Description
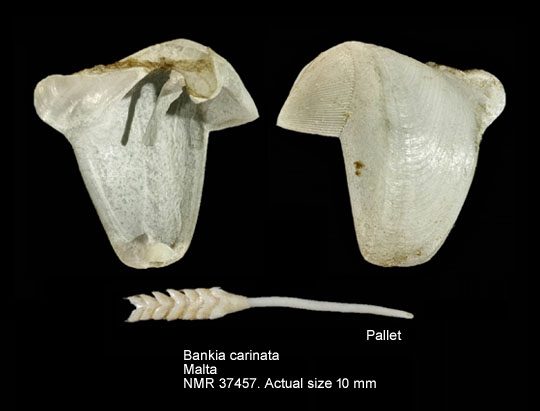
Bankia carinata belongs to the family Teredinidae (shipworms), which are highly modified mollusks, hardly recognizable as bivalves, and adapted for boring into wood. The shell is reduced to two small, ridged valves, which cover the head and are used for grinding and tearing wood fibers. The valves are very similar to those in Teredo. The shell of Bankia carinata, like those of other species, has three subglobular lobes. The smallest of these is the auricle, which is semicircular and subtriangular. The interior of the shell has a long, curved process (styloid apophysis). The body is naked and elongated, and ends with two siphons, protected by elaborate calcareous structures called pallets. The pallets are elaborate plume-like structures, consisting of nested cones at the end of a long stalk about 0.8 mm in length. The cones are funnel-shaped, shallow-cupped, with blunt, smooth edges. The cups are crowded at the tip of the pallet and covered with a cap of periostracum. Variability in the shells and pallets has led to many specific names (e.g. Turner 1966), which are now treated as synonyms. Description from: Turner 1966; Turner 1971; Abbott 1974; Coan et al. 2000. Identification of shipworms to species requires a specialist.
Genetic sequencing of B, carinata from the Mediterranean and the Caribbean has shown strong differences between populations, suggesting that 'B. carinata' could be a complex of several species (Borges et al. 2012). Gene sequences of Bankia carinata from different locations clustered with morphologically divergent genera, with specimens from Japanese tsunami debris grouped with Teredora princesae, a Pacific pelagic species, while Mediterranean B. carinata clustered with Lyrodus pedicellatus, and B. carinata (Treneman, et al. 2018).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Mollusca | |
| Class: | Bivalvia | |
| Subclass: | Heterodonta | |
| Order: | Myoida | |
| Superfamily: | Pholadoidea | |
| Family: | Teredinidae | |
| Genus: | Bankia | |
| Species: | carinata |
Synonyms
Teredo minima (Blainville, 1828)
Teredo stutchburyi (Blainville, 1828)
Teredo bipalmata (Delle Chiaje, None)
Xylotrya philippii (Gray, 1851)
Bankia oryzaformis (Sivickis, 1928)
Bankia kuronunii (Roch, 1929)
Bankia segaruensis (Roch, 1929)
Nausitora kamiyai (Roch, 1929)
Nausitora orientalis (Roch, 1929)
Bankia nakazawai (Kuronuma, 1931)
Bankia consularis (Moll, 1935)
Bankia syriaca (Roch, 1936)
Bankia caribbea (Clench & Turner, None)
Bankia edmondsoni (Nair, 1956)
Potentially Misidentified Species
Caribbean, Pacific Panama
Bankia destructa
Caribbean, Pacific Panama
Bankia fimbriatula
Caribbean and West Atlantic
Bankia fosteri
southern Caribbean
Bankia zeteki
Caribbean, Pacific Panama
Ecology
General:
Shipworms dig long burrows in submerged wood in marine environments. They burrow by rocking and abrading the wood fibers. The mantle covers most of the length of the body and secretes a calcareous lining along the interior of the burrow. They normally have their anterior end with head and shells inside the burrow, and their siphons protruding outwards. The pallets plug the burrow when the siphons are retracted (Barnes 1983).
The shipworm Bankia carinata breeds in warm-temperate to tropical waters, extending north into the southern half of Japan and the Mediterranean Sea (Turner 1966; Tsunoda et al. 1999; Borges et al. 2014b). In the Mediterranean, it was limited to temperatures of 9–40 C, and salinities of 35 to 39 PSU (Borges et al. 2014b). Shipworms may obtain most of their nutrition from plankton (Paalvast and van der Velde 2013), but some comes from wood, which consists largely of cellulose. Symbiotic bacteria fix nitrogen, essential for protein synthesis (Turner and Johnson 1971; Barnes 1983). Bankia carinata occurs in drift logs, human-disposed wood, and in the tropics, the wood of mangroves (MacIntosh et al. 2014).
Food:
Phytoplankton, Wood
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Coarse Woody Debris | None |
| General Habitat | Marinas & Docks | None |
| General Habitat | Mangroves | None |
| General Habitat | Vessel Hull | None |
| Salinity Range | Euhaline | 30-40 PSU |
| Tidal Range | Subtidal | None |
| Vertical Habitat | Epibenthic | None |
Life History
Shipworms are protandrous hermaphrodites, beginning life as male and transforming to female, but they have no capacity for self-fertilization. Males release sperm into the water column, which fertilizes eggs for the female. Bankia carinata is oviparous, so the eggs are fertilized and develop in the water column (Turner and Johnson 1971; MacIntosh et al. 2014). The shipworms reach male maturity at lengths of 2.5 to 4 mm, and female maturity at 8–10 mm. Individuals of 30 mm could release 15,000 eggs, while females of 100 mm had fecundities of 3.0 x 10^6 eggs (MacIntosh et al. 2014).
Tolerances and Life History Parameters
| Maximum Depth (m) | 7,488 | Banda Sea, Indonesia (Turner 1966). |
| Minimum Temperature (ºC) | 9 | Field (Borges et al. 2014b, European range) |
| Maximum Temperature (ºC) | 30 | Field (Borges et al. 2014b, European range) |
| Minimum Salinity (‰) | 35 | Field (Borges et al. 2014b, European range) |
| Maximum Salinity (‰) | 39 | Field (Borges et al. 2014b, European range) |
| Minimum Duration | 11 | From hatching to pediveliger stage, assumed to be capable of settling (Bair 1956, cited by Turner and Johnson 1971). |
| Minimum Length (mm) | 2.5 | MacIntosh et al. 2014 |
| Maximum Length (mm) | 103 | MacIntosh et al. 2014 |
General Impacts
The shipworm Bankia carinata is an important woodborer in subtropical and tropical regions, but probably has no impact in West Coast waters. In the Gulf and Caribbean, it is one of many species of native and cryptogenic shipworms.
Regional Distribution Map
| Bioregion | Region Name | Year | Invasion Status | Population Status |
|---|---|---|---|---|
| SEP-Z | 1989 | Non-native | Established |
Occurrence Map
| OCC_ID | Author | Year | Date | Locality | Status | Latitude | Longitude |
|---|
References
Abbott, R. Tucker (1974) American Seashells, Van Nostrand Reinhold, New York. Pp. <missing location>Barnes, Robert D. (1983) Invertebrate Zoology, Saunders, Philadelphia. Pp. 883
Borges, L. M. S.; Sivrikaya, H.; Le Roux, A.; Shipway, J. R.; Cragg, S. M.; Costa, F. O. (2012) Investigating the taxonomy and systematics of marine wood borers (Bivalvia : Teredinidae) combining evidence from morphology, DNA barcodes and nuclear locus sequences, Invertebrate Systematics 26: 572-582
Borges, Luísa M. S.; Merckelbach, Lucas M.; Sampaio, Íris; Cragg, Simon M. (2014b) Diversity, environmental requirements, and biogeography of bivalve wood borers (Teredinidae) in European coastal waters, Frontiers in Zoology 11(13): Published online
Coan, E. V.; Scott, Paul H. (1997) Checklist of the marine bivalves of the Northeastern Pacific Ocean, Santa Barbara Museum of Natural History Contributions 1: 1-28
Coan, Eugene V.; Valentich-Scott, Paul; Bernard, Frank R. (2000) Bivalve Seashells of Western North Ameira, Santa Barbara Museum of Natural history, Santa Barbara CA. Pp. <missing location>
Cruz, Manuel P. (1996) [Contribution to the knowledge of wood-boring organisms of the island of Baltra, Galàpagos Archipelago, Ecuador], Acta Oceanografica del Pacifico 8: 75-85
Cruz, Manuel; Torres, Glasys; Villamar, Felicia (1989) Comparative study of the woodboring bivalves of the more resistant woods (Laurel, 'Moral', Cow Tree) and the more vulnerable (Mangrove) on the coast of Ecuador], Acta Oceanografica del Pacifico 5(1): 49-55
Harvard Museum of Comparative Zoology 2008-2021 Museum of Comparative Zoology Collections database- Malacology Collection. <missing URL>
Ibrahim, J. V. (1981) Season of settlement of a number of shipworms (Mollusca: Bivalvia) in six Australian harbors., Australian Journal of Marine and Freshwater Research 32: 591-604
MacIntosh H.; de Nys, R.; Whalen, S. (2014) Contrasting life histories in shipworms: Growth, reproductive development and fecundity, Journa of Experimental Marine Biology and Ecology 459: 80-86
Museum of Comparative Zoology 2008-2015 Invertebrate Zoology Collections Database http://mczbase.mcz.harvard.edu/SpecimenSearch.cfm. <missing URL>
Paalvast, Peter; van der Velde, Gerard (2013) What is the main food source of the shipworm Teredo navalis? A stable isotope approach, Journal of Sea Research 80: 58-60
Raveendran, T. V.; Wagh, A. B. (1991) Distribution and growth of wood-borers in Bombay offshore waters., Indian Journal of Marine Science 20: 143-146
Sen, Selim; Sivrikaya, Huseyin; Yalcin, Mesut; Bakir, Ahmet Kerem; Öztürk, Bilal (2010) Fouling and boring organisms deteriorating various European and tropical woods on the Turkish coast line, African Journal of Biotechnology 9(17): 2566-2573
Treneman, N.C.; Borges L.M.S.; Shipway, J.R.;Raupach M.J.; Altermark, B. (2018) A molecular phylogeny of wood-borers (Teredinidae) from Japanese Tsunami Marine Debris, Aquatic Invasions 13(1): 101-112
Tsunoda, Kunio (1979) Ecological studies of shipworm attack on wood in the sea water log storage site, Wood Research: Bulletin of the Wood Research Institute Kyoto University 65: 11-53
Turner, R. D. (1971) Identification of marine wood-boring molluscs, In: Jones, E. G.; Eltringham, S. E(Eds.) Marine borers, fungi and fouling organisms of wood.. , Paria. Pp. 17-64
Turner, R. D.; Johnson, A. C. (1971) Biology of marine wood-boring molluscs, In: Jones, Evan Benjamin Gareth; Eltringham, Stewart Keith(Eds.) Marine borers, fungi and fouling organisms of wood.. , Paris. Pp. 259-301
Turner, Ruth D. (1966) A survey and illustrated catalogue of the Teredinidae (Mollusca: Bivalvia), The Museum of Comparative Zoology, Harvard University, Cambridge. Pp. <missing location>
Turner, Ruth D. (1971) Marine Borers, Fungi, and Fouling Organisms of Wood, Organisation for Economic Co-operation and Development, Paris. Pp. <missing location>
WoRMS Editorial Board 2022-2024 World Register of Marine Species. https://www.marinespecies.org
https://doi.org/10.14284/170