Invasion History
First Non-native North American Tidal Record: 1983First Non-native West Coast Tidal Record:
First Non-native East/Gulf Coast Tidal Record: 1983
General Invasion History:
Lyrodus medilobatus is a shipworm originally described from Hawaii. Its native distribution is unknown, but it now has a wide distribution in the Indo-Pacific from Hawaii and the Galapagos to New Zealand, Australia, and India (Turner 1966; McKoy 1980; Santhakumaran 1986; Museum of Comparative Zoology 2008-2012). In the Atlantic where we consider it introduced, this shipworm has been collected in Bermuda, the US Virgin Islands, and Florida, as scattered specimens (Mikkelsen et al. 1995; Museum of Comparative Zoology 2008-2012). However, established populations are still unknown in Atlantic waters.
North American Invasion History:
Invasion History on the East Coast:
We have found four records of the Indo-Pacific shipworm Lyrodus medilobatus in the Western Atlantic. Two were in the Indian River Lagoon, Florida, in the Fort Pierce and St. Lucie Inlets, but considered without viable populations (Mikkelsen et al. 1995). It was also found in the US Virgin Islands, off of Frederiksted on St. Croix (1906, MCZ 278037, Museum of Comparative Zoology 2009), and Bermuda (date unknown, Turner 1966) but with no reports of established populations. Extensive surveys for shipworms in US naval ports by the William Clapp Laboratories, sponsored by the US Navy from the 1920s to the 1960s, had not identified this clam (Brown 1953; Wallour 1962; Turner 1966).
Invasion History Elsewhere in the World:
Lyrodus medilobatus was collected on wood panels in Salinas, Ecuador in 1987. It was relatively uncommon but occurred on four different kinds of wood (Cruz et al. 1989).
Description
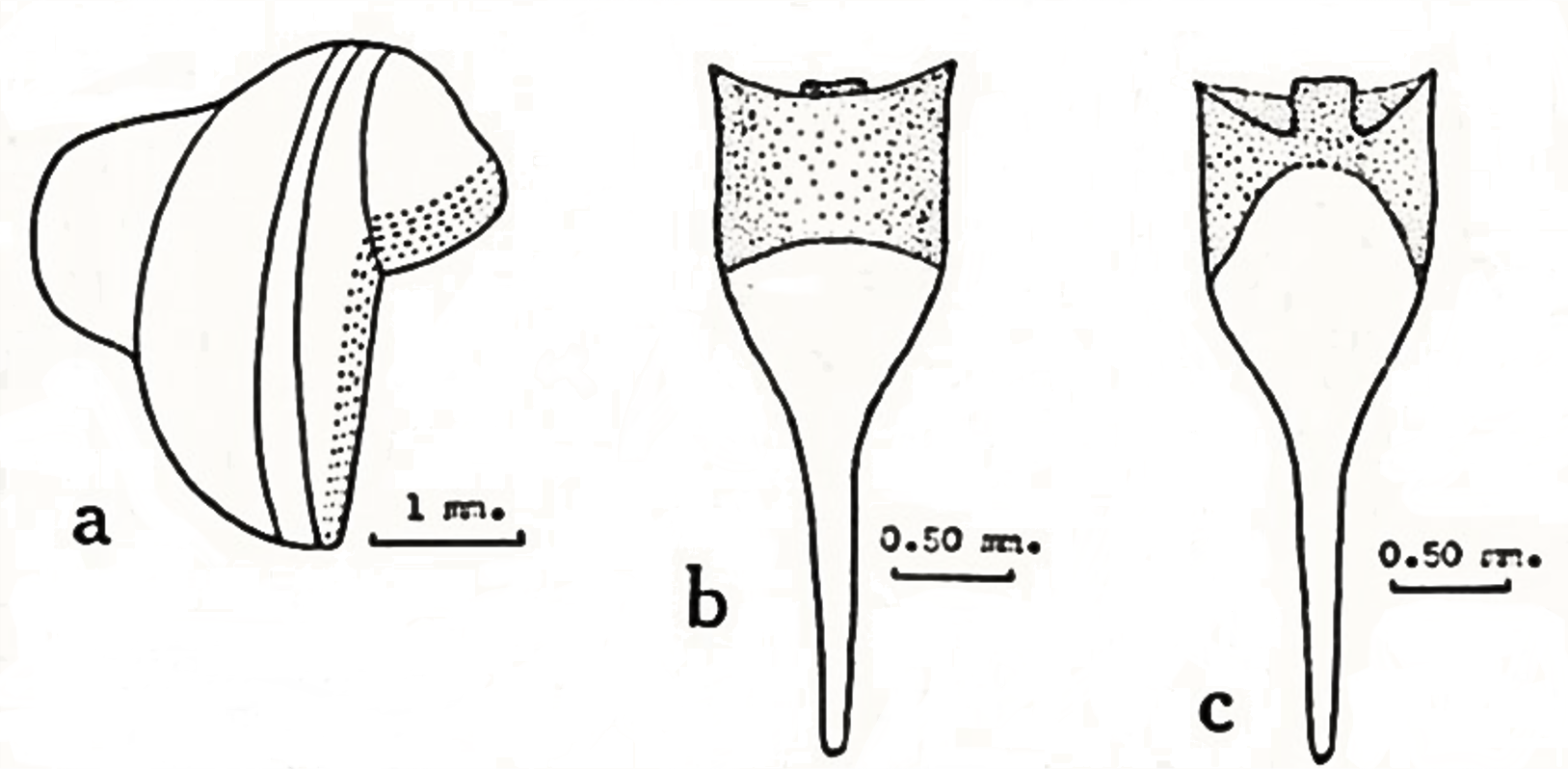
Lyrodus medilobatus belongs to the family Teredinidae (shipworms), which are highly modified mollusks, hardly recognizable as bivalves, and adapted for boring into wood. The shell is reduced to two small, ridged valves covering the head, and are used for grinding and tearing wood fibers. The body is naked and elongated and ends with two siphons, protected by elaborate calcareous structures called pallets.
In the genus Lyrodus, the shell resembles that of the Naval Shipworm (Teredo navalis), but is smaller and more finely sculptured. The pallets have a calcareous base, which is conical distally with a brown to black cap made of periostracum. The outline resembles that of the stem and cup of a wineglass. The outer distal margin of the cap is nearly straight, but the inner margin is U-shaped, with a prominent median lobe (Turner 1966; Santhakumaran 1986).
Potentially misidentified species — The diversity of shipworms in tropical waters is very great. Many are now widely distributed in the Atlantic, Pacific, and Indian Oceans, largely because of shipping. The species listed have been reported in Florida and Caribbean waters.
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Mollusca | |
| Class: | Bivalvia | |
| Subclass: | Heterodonta | |
| Order: | Myoida | |
| Superfamily: | Pholadoidea | |
| Family: | Teredinidae | |
| Genus: | Lyrodus | |
| Species: | medilobatus |
Synonyms
Teredo medilobata (Edmondson, 1942)
Potentially Misidentified Species
Native to Caribbean, introduced to the East Pacific
Bankia gouldi
Native to NW Atlantic introduced to the East Pacific
Lyrodus bipartitus
Cosmopolitan, tropical, subtropical
Lyrodus floridanus
Caribbean, native?
Lyrodus pedicellatus
Cosmopolitan, tropical, subtropical, warm-temperate, introduced in the northeast Pacific
Teredo bartschi
Cosmopolitan, introduced in northeast Pacific
Teredo clappi
Cosmopolitan, tropical, subtropical
Teredo furcifera
Cosmopolitan, tropical, subtropical
Teredo johnsoni
Cosmopolitan, tropical, subtropical
Teredo navalis
Origin unknown, introduced in the northwest Atlantic, northeast Pacific
Ecology
General:
Shipworms dig long burrows in submerged wood in marine environments, and normally have their anterior end with head and shell inside the burrow, with their siphons protruding. The animal burrows by rocking and abrading the wood fibers. The mantle covers most of the length of their body and secretes a calcareous lining along the interior of the burrow. The pallets plug the burrow when the siphons are retracted (Barnes 1983).
Lyrodus medilobatus is known from fixed wood structures and panels, and from mangrove habitats in tropical and subtropical climates (Turner 1966; McKoy 1980). It is absent from the southernmost parts of Australia (Victoria and Tasmania) and is limited to the northern peninsula of the North Island of New Zealand (north of 37°S latitude, McKoy 1980). This shipworm seems to be somewhat tolerant of low salinities and was collected at two estuarine sites at 7 and 10 PSU (McKoy 1980).
Food:
Wood, phytoplankton
Trophic Status:
Herbivore
HerbHabitats
| General Habitat | Coarse Woody Debris | None |
| General Habitat | Marinas & Docks | None |
| General Habitat | Vessel Hull | None |
| General Habitat | Mangroves | None |
| Salinity Range | Polyhaline | 18-30 PSU |
| Salinity Range | Euhaline | 30-40 PSU |
| Salinity Range | Mesohaline | 5-18 PSU |
| Tidal Range | Subtidal | None |
| Tidal Range | Low Intertidal | None |
| Vertical Habitat | Epibenthic | None |
Life History
Shipworms are protandrous hermaphrodites, beginning their life as male and subsequently transforming to female, but have no capacity for self-fertilization. Males release sperm into surrounding seawater, which fertilizes the eggs that are brooded in the gills of females. Larvae are retained in the gills to the veliger stage. In some species, such as Teredo navalis and Lyrodus massa they are released in the early (straight-hinge) stages, and spend several weeks in the plankton, while in others (T. bartschi, T. furcifera, Lyrodus pedicellatus) they are released in an advanced stage as pediveliger and spend only a few days in the plankton. We do not know the period of the planktonic stage in L. medilobatus. The larvae settle in the pediveliger stage, and then rapidly metamorphose and begin boring into wood within 2–3 days. They soon develop a calcified shell, pallets, and burrow lining (Turner and Johnson 1971). Shipworms may obtain some or most of their nutrition from plankton, but some comes from wood, which consists largely of cellulose (Paalvast and van der Velde 2013). Symbiotic bacteria fix nitrogen, essential for protein synthesis (Turner and Johnson 1971; Barnes 1983).
Tolerances and Life History Parameters
| Minimum Salinity (‰) | 7 | McKoy 1980 |
| Broad Temperature Range | None | Subtropical-Tropical |
| Broad Salinity Range | None | Mesohaline-Euhaline |
General Impacts
The shipworm Lyrodus medilobatus is an abundant wood-borer in mangroves and wood structures in the tropics. However, it has not become established where it has been introduced in the Western Atlantic, and has no reported impacts. It is established but uncommon in the Galapagos Islands and mainland Ecuador (Cruz 1989; Cruz 1996).
Regional Distribution Map
Non-native
Native
Cryptogenic
Failed
Occurrence Map
References
Barnes, Robert D. (1983) Invertebrate Zoology, Saunders, Philadelphia. Pp. 883Brown, Dorothy J. (1953) <missing title>, Report No. 8511 William F. Clapp Laboratories, Inc., Duxbury, Massachusetts. Pp. <missing location>
Carlton, James T.; Ruckelshaus, Mary H. (1997) Nonindigenous marine invertebrates and algae of Florida, In: Simberloff, Daniel, Schmitz, Don C., Brown, Tom C.(Eds.) Strangers in Paradise: Impact and Management of Nonindigenous Species in Florida. , Washington, D.C.. Pp. 187-201
Cruz, Manuel P. (1996) [Contribution to the knowledge of wood-boring organisms of the island of Baltra, Galàpagos Archipelago, Ecuador], Acta Oceanografica del Pacifico 8: 75-85
Cruz, Manuel; Torres, Glasys; Villamar, Felicia (1989) Comparative study of the woodboring bivalves of the more resistant woods (Laurel, 'Moral', Cow Tree) and the more vulnerable (Mangrove) on the coast of Ecuador], Acta Oceanografica del Pacifico 5(1): 49-55
Harvard Museum of Comparative Zoology 2008-2021 Museum of Comparative Zoology Collections database- Malacology Collection. <missing URL>
Ibrahim, J. V. (1981) Season of settlement of a number of shipworms (Mollusca: Bivalvia) in six Australian harbors., Australian Journal of Marine and Freshwater Research 32: 591-604
McKoy, J. L (1980) Distribution of shipworms (Bivalvia: Teredinidae) in the New Zealand region, New Zealand Journal of Marine and Freshwater Research 14(3): 263-275
Mikkelsen, Paula M., Mikkelsen, Paul S., Karlen, David J. (1995) Molluscan biodiversity in the Indian River Lagoon, Florida, Bulletin of Marine Science 57(1): 94-127
Paalvast, Peter; van der Velde, Gerard (2013) What is the main food source of the shipworm Teredo navalis? A stable isotope approach, Journal of Sea Research 80: 58-60
Santhakumaran, L. N. (1986) Lyrodus medilobata (Edmondson) (Mollusca: Teredinidae): a new record from Indian rivers., Mahasagar 19: 271-273
Turner, R. D.; Johnson, A. C. (1971) Marine Borers, Fungi, and Fouling Organisms of Wood, Organisation for Economic Co-operation and Development, Paris. Pp. 259-301
Turner, Ruth D. (1966) A survey and illustrated catalogue of the Teredinidae (Mollusca: Bivalvia), The Museum of Comparative Zoology, Harvard University, Cambridge. Pp. <missing location>
Turner, Ruth D. (1971) Marine Borers, Fungi, and Fouling Organisms of Wood, Organisation for Economic Co-operation and Development, Paris. Pp. <missing location>
Wallour, Dorothy Brown (1960) Thirteenth progress report on marine borer activity in test boards operated during 1959, William F. Clapp Laboratories, Duxbury, Massachusetts. Pp. 1-41