Invasion History
First Non-native North American Tidal Record: 1897First Non-native West Coast Tidal Record: 1897
First Non-native East/Gulf Coast Tidal Record:
General Invasion History:
Ciona robusta was formerly considered to be Ciona intestinalis. Morphological, ecological, and genomic data indicated that populations of C. intestinalis from the Mediterranean and the Pacific Ocean ('Sp. A') were similar to each other, but differed from the typical C. intestinalis ('Sp. B') from the coast of Northern Europe and the East Coast of North America (Dybern 1965; Boffelli et al. 2004; Suzuki et al. 2005; Caputi et al. 2007; Nydam and Harrison 2007; Nydam and Harrison 2010). Genetic analyses indicate that the two species have been largely isolated for 3-5 million years (Nydam and Harrison 2007; Roux et al. 2013). Brunetti et al. (2015) used morphological and genetic data to identify 'Sp. A' as C. robusta described by Hoshino and Tokioka (1967) from Japan.
Tentatively, we assign the Northwest Pacific as the native region of C. robusta (Gretchen Lambert, personal communication). Ciona robusta was collected in Yokohama, Japan, in 1902 (Hoshino and Nishikawa 1985), and has been regarded as an introduced species in Japan and Korea (Asakura 1992; Lee and Shin 2014; Pyo et al. 2012). It was collected very early in the Mediterranean (Savigny 1816, Heller 1875, Traustedt 1882, Roule 1884, all cited by Hoshino and Nishikawa 1985), but that may reflect the early development of oceanic trade between Europe and Asia. Further genomic studies may revise our ideas of C. robusta's origin and invasion history.
Ciona robusta was first collected in Australia in 1878 (Kott 1990), followed by the West Coast of North America in 1897 (San Diego, Wheeler 1897, cited by Carlton 1979), Hawaii in 1933 (Edmondson 1933, cited by Carlton and Eldredge 2009), Atlantic South America in 1945 (Argentina, Orensanz et al. 2002), New Zealand in 1950 (Cranfield et al. 1998), South Africa in 1955 (Millar 1955, cited by Monniot et al. 2001), and Pacific South America in 2002 (Castilla et al. 2005). Ciona robusta is a widespread and abundant fouling organism in harbors in the warmer parts of the world, while C. intestinalis seems better adapted to cooler regions (Dybern 1967; Marin et al. 1987; Caputi et al. 2007; Zhan et al. 2010).
North American Invasion History:
Invasion History on the West Coast:
Molecular identifications of C. robusta as 'C. intestinalis sp. A' have been made on specimens from Half Moon Bay, Sausalito (in San Francisco Bay; Nydam and Harrison 2007), and Santa Barbara Harbor (Caputi et al. 2007; Zhan et al. 2010), California. We assume, provisionally, that all the established West Coast populations are C. robusta. However, additional genetic sampling is desirable. Ciona robusta (up to now, reported as C. intestinalis) was first reported by Wheeler 1897 (cited by Carlton 1979) from San Diego Bay, 'growing in masses on pilings at Coronado' (Carlton 1979). It was subsequently found in San Diego by many other authors (Morgan 1905; Ritter and Forsyth 1917; Morgan 1941; Farmer 1964, all cited by Carlton 1979; Lambert and Lambert 1998; Lambert and Lambert 2003). This tunicate seems to have spread first to harbors with more shipping or boat traffic, including: San Francisco Bay (in 1932, Rodholm 1932, cited by Carlton 1979); Los Angeles-Long Beach (Barnard 1958); and Newport Bay (MacGinitie 1939, cited by Carlton 1979). Then it spread to smaller, more isolated harbors, including: Mission Bay (Farmer 1964, cited by Carlton 1979); Morro Bay (in 1968, Needles 2007); Santa Barbara Harbor, King Harbor (in 1970, Fay and Johnson 1971, cited by Carlton 1979); and Monterey Harborf (in 1974, Haderlie and Donat 1978). By 2000-2010, C. robusta was recorded in every sampled mainland harbor from San Diego to Bodega Bay, although its appearance was sporadic in some (Lambert and Lambert 1998; Fairey et al. 2002; Lambert and Lambert 2003; de Rivera et al. 2005a). In San Francisco Bay, it has been found in Lake Merritt, Central bay, and South Bay (Carlton 1979; Nydam and Harrison 2007; Chang 2009; Foss 2009), but we have not found reports from San Pablo Bay.
The occurrence of C. robusta north of Bodega Bay is uncertain. In Humboldt Bay, Boyd et al. reported only C. intestinalis, while Fairey et al. reported both C. intestinalis and C. savignyi. (Boyd et al. 2002; Fairey et al. 2002). However, it has not been found on SERC fouling plates in Humboldt Bay (Ruiz et al., unpublished data). There are old records from Haida Gwaii (Queen Charlotte Islands) and Vancouver Island, British Columbia and the San Juan Archipelago, Washington (Huntsman 1912, Fraser 1932, all cited by Carlton 1979). These records are historical and the current northern range edge of this species is unknown (Carlton 1979; Gretchen Lambert, personal communication).
Invasion History in Hawaii:
Ciona robusta (then identified as C. intestinalis) was recorded from Pearl and Honolulu harbors, Oahu by 1933 (Edmondson 1933, cited by Carlton and Eldredge 2009). It was found on the hull of a ship (USS Dobin) in Pearl Harbor in 1940 (Abbott 1941, cited by Carlton and Eldredge 2009) and has also been found in Kaneohe Bay (Abbott 1997, cited by Carlton and Eldredge 2009). It should be noted, that to our knowledge, molecular identifications have not been made.
Invasion History Elsewhere in the World:
As noted above, we are considering Ciona robusta native to the Northwest Pacific and introduced in European waters. This tunicate was collected in Egyptian waters as early as 1816, and was widespread in the Mediterranean Sea by the late 1800s, based on the synonyms listed by Hoshino and Nishikawa (1985). Its occurrence in the Black Sea is uncertain, since the only specimens in a molecular analysis were assigned to the cryptic 'Species D' (Zhan et al. 2010). On the Atlantic Coast of Europe, C. robusta co-occurs with C. intestinalis in Plymouth Harbor, England and Brittany, France (Caputi et al. 2007; Zhan et al. 2010; Nydam and Harrison 2011; Sato et al. 2012). Ciona robusta's distribution in this region is highly localized, and more sporadic, while the range of C. intestinalis is more continuous. In 2007, purebred individuals were found at 3 locations each in southwest England and Brittany, but by 2009 only hybrids were found, and at only two locations each (Nydam and Harrison 2011). Nydam and Harrison (2011) suggested that shipping or Pacific Oyster (Crassostrea gigas) introductions were possible vectors for these introductions.
'C. intestinalis' was reported from Durbin, South Africa on the Indian Ocean in 1955 (Millar 1955, cited by Monniot et al. 2001), from Saldanha Bay on the Atlantic side in 1962, and at many South African ports by 2001 (Monniot et al. 2001; Mead et al. 2011b; Rius et al. 2014). Populations from Cape Town have been identified as C. robusta (Zhan et al. 2010). In the Southwest Atlantic, 'C. intestinalis' was first collected in 1945 in Mar del Plata, Argentina (Orensanz et al. 2002) and was found in San Antonio Este and Puerto Madryn in 2005 (Schwindt et al. 2014). It was collected in Santos, Brazil by 1958 (Millar 1958), and has been found sporadically between Santos and Rio de Janeiro (de Oliveira Marins et al. 2009; da Rocha and Bonnet 2009). We presume that these records refer to C. robusta, but molecular confirmations are needed.
In Australia, the first record of 'C. intestinalis' is from Port Jackson, Sydney, New South Wales in 1878 (Kott 1990; Keough and Ross 1999). It was subsequently found at many ports around the southern coast of Australia, including: Fremantle and Albany in Western Australia (Hartmeyer and Michaelsen 1928, cited by Kott 1990); Port Adelaide, South Australia; Hobart, Tasmania (Kott 1952, cited by Kott 1990); and Port Philip Bay, New South Wales (in 1958, Millar 1966, cited by Keough and Ross 1999). Specimens from Port Lincoln, South Australia have been identified as C. robusta ('Species A.', Zhan et al. 2010). In New Zealand, 'Ciona intestinalis' was first reported in 1950 (Brewin 1950) in Lyttleton Harbour. It is known from the harbors of Lyttleton, Napier and Nelson (Cranfield et al. 1998; Inglis et al. 2006c; Inglis et al. 2006f). Specimens from Nelson were identified as C. robusta by molecular methods (Species A.', Smith et al. 2010).
On the other side of the Pacific, ‘C. intestinalis' was reported, very early, from the Straits of Magellan, Chile (Traustedt 1885, cited by Dybern 1965; Castilla et al. 2005). Experiments suggest that C. intestinalis is unlikely to become established in the Magellan region (Madariaga et al. 2014). However, since 2002, populations have become established in bays from the Peruvian border to central Chile (14-40°S, Castilla et al. 2005; Sanamyan and Schories 2005; Madariaga et al. 2014). Chilean populations are presumed to be C. robusta, but have not been identified by molecular methods.
Description
Ciona 'intestinalis' ws formerly considered to be widely distributed in the Atlantic, Mediterranean, and North and South Pacific (Van Name 1912; Van Name 1945; Dybern 1965; Carlton 1979; Hoshino and Nishikawa 1985; Nishikawa 1991). Recent morphological and genomic work has shown that ‘'Ciona intestinalis'’, which was described from Sweden by Linneaus in 1767, is a complex of species (Caputi et al. 2007; Brunetti et al. 2015; Pennati et al. 2015). Genomic studies revealed genetic differences between European populations, with Mediterranean populations showing strong affinities with US West Coast and most Asian populations, while diverging from Northern European and East Coast populations (Boffelli et al. 2004; Suzuki et al. 2005; Caputi et al. 2007; Nydam and Harrison 2007; Nydam and Harrison 2010). Caputi et al. (2007) identified the Mediterranean-Pacific form as 'Ciona intestinalis sp. A', and noted that it was more genetically homogeneous than the North Atlantic 'Ciona intestinalis sp. B', which was more geographically structured. Hybrids of the two species were asymmetrically infertile. C. intestinalis can be fertilized by C. robusta sperm, but the reverse cross had very low fertility (Caputi et al. 2007; Malfant et al. 2017). Hybrids are rare in the wild (Bouchemousse et al. 2016b; Malfant et al. 2017). Populations of the two species were found to overlap in Plymouth Harbour, England, and could be separated by morphology (tubercles on the tunic near the siphon, in species B) and coloration (Sato et al. 2012). These tubercles had been described previously in a Japanese form, given the name Ciona robusta (Hoshino and Tokioka 1967), but later synonymized with C. intestinalis (Hoshino and Nishikawa 1985). Brunetti et al. (2015) found that the tubercles could be detected over the whole tunic, in 'species A', using appropriate lighting, histology, and 3-D reconstruction, in combination with genetic information. They identified 'species A' as C. robusta. However pigmentation and tubercles alone were insufficient to separate the two species. Morphometric features of larvae are the most decisive morphological feature for separating the two species (Pennati et al. 2015).
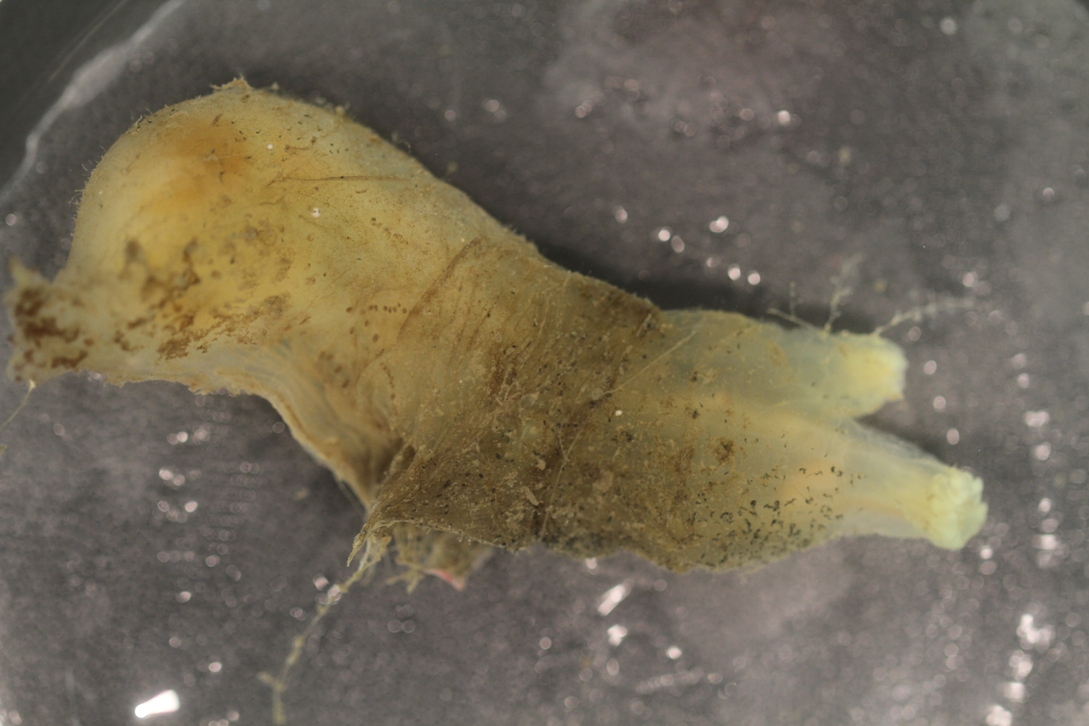
Ciona robusta has a transparent or translucent tunic. Much of the tunic is soft, flexible, and gelatinous, except for the posterior end where it can be tough, mostly opaque, white or yellowish-white. The muscle bands and organs are often visible beneath the tunic. The body is white or off-white. The siphons are short and directed forward, with the oral siphon larger than the atrial siphon. The oral siphon has 8 lobes, while the atrial siphon has 6 lobes. Both siphons lack bright pigment, although a pale white or yellow tinge is sometimes visible. Tubercles on the tunic are usually most visible near the siphons, but are scattered over the surface, though lighting, histological sectioning, and 3-D imaging may be needed to see all of them. There are 5-7 conspicuous longitudinal muscle bands on each side that extend nearly the entire length of the body (Hoshino and Tokioka 1967; Sato et al. 2012; Brunetti et al. 2015). Ciona robusta can reach a length of 210 mm long, but a more typical maximum is 100-120 mm (Hoshino and Tokioka 1967; Hoshino and Nishikawa 1985, specimens from Naples).
Ciona robusta is very similar in appearance to C. savignyi, but there are a few notable morphological differences. Ciona robusta never has white pigmented flecks or spots in its body wall while C. savignyi always has these spots. The number of tentacles around the oral siphon is variable in both species but generally C. robusta has more (n>50) tentacles than C. savignyi (Hoshino and Nishikawa 1985). Ciona robusta has an endostylar appendage while C. savignyi does not (Hoshino and Nishikawa 1985). The pair of pharyngeo-epicardiac openings in C. robusta is usually very small and located near its base while in C. savignyi these openings are located close to the esophageal opening (Hoshino and Nishikawa 1985).
Sato et al. (2012) found C. robusta (at that time it was known as C. intestinalis species 'A') and C. intestinalis ('species B') living sympatrically in Plymouth Harbor, England, as indicated by genetic analysis. Ciona robusta specimens had little pigment on the margins of the oral siphons, while most C. intestinalis tunicates had strong yellow pigmentation on the margins. Most C. robusta had small tubercles on the tunic, while most C. intestinalis specimens lacked them. The two species also tended to differ in the color of papillae on the gonoducts, with more intense coloration in most C. intestinalis specimens. These differences were heritable in culture, and intermediate in hybrids (Sato et al. 2012). As larvae, C. robusta have a shorter pre-oral lobe, a shorter and wider body, and a long ocellus-tail distance, compared to C. intestinalis (Pennati et al. 2015). Additional sampling will be needed to determine whether these differences are consistent over regions and seasons (Pennati et al. 2015).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Chordata | |
| Subphylum: | Tunicata | |
| Class: | Ascidiacea | |
| Order: | Phlebobranchia | |
| Family: | Cionidae | |
| Genus: | Ciona | |
| Species: | robusta |
Synonyms
Ascidia intestinalis ( Linnaeus, 1767)
Ascidia canina (Mueller, 1776)
Potentially Misidentified Species
Ciona intestinalis is native to the North Atlantic, and distinguishable from C. robusta by genetics, and to varying degrees by pigmentation, the presence of tubercles on the tunic, and larval morphology (Caputi et al. 2007; Nydam and Harrison 2007; Zhan et al. 2010; Sato et al. 2012; Brunetti et al. 2015; Pennati et al. 2015). It has been introduced to the Yellow and Bohai Seas (Zhan et al. 2010) and to Iceland (Svavarsson and Dungal 2008, cited by Thorarinsdottir et al. 2014).
Ciona savignyii
Ciona savignyii is a Northwest Pacific native, introduced to the West Coast (Lambert and Lambert 1998; Lambert 2003) and New Zealand (Smith et al. 2010).
Ciona Species C
Ciona Species C is an undescribed species identified by molecular means from the Mediterranean (Zhan et al. 2010).
Ciona Species D
Ciona Species D is an undescribed species identified by molecular means from the Black Sea (Zhan et al. 2010).
Ecology
General:
Life History – Ciona robusta is a vase-shaped, solitary tunicate, attached at its base to a substrate. It has two openings or siphons, an oral and an atrial siphon. Water is pumped in through the oral siphon, where phytoplankton and detritus is filtered by the gills and passed on mucus strings to the stomach and intestines. Waste is then expelled in the outgoing atrial water.
Solitary ascidians are hermaphroditic, meaning that both eggs and sperm are released to the atrial chamber. Eggs may be self-fertilized or fertilized by sperm from nearby animals, but many species have a partial block to self-fertilization. In C. robusta from the Tyrrhenian Sea, Italy, both self- and non-self-fertilization took place (Caputi et al. 2015). Depending on the species, eggs may be externally or internally fertilized. In external fertilizers, such as C. robusta, eggs and sperm are released through the atrial siphon into the surrounding water column where fertilization takes place. Fertilized eggs hatch into a tadpole larva with a muscular tail, notochord, eyespots, and a set of adhesive papillae. The lecithotrophic (non-feeding, yolk-dependent) larva swims briefly before settlement. Swimming periods are usually less than a day and some larvae settle immediately after release. Once settled, the tail is absorbed, the gill basket expands, and the tunicate begins to feed by filtering (Barnes 1983).
Ciona robusta is an abundant fouling organism on pilings, buoys, aquaculture facilities, and rocky shores (Haderlie et al. 1978; Hoshino and Nishikawa 1985; Castillo et al. 2005; Dumont et al. 2011). Ciona robusta appears to differ from its cryptic congener C. intestinalis in its temperature and salinity tolerance, with C. robusta associated with Mediterranean and subtropical climates and higher salinities, while C. intestinalis tolerates more boreal climates and extends into lower salinities, including the Baltic Sea and Norwegian estuaries (Dybern 1965; Marin et al. 1987; Madariaga et al. 2014). The lowest average winter temperature tolerated by C. robusta was ~3°C in the Lagoon of Venice (Dybern 1965), while the lowest salinity tolerated was 21-25 PSU (Marin et al. 1987; Madariaga et al. 2014). By contrast, C. intestinalis survived temperatures below 0°C and salinities as low as 9 PSU (Dybern 1965; Dybern 1967). Ciona robusta is frequently rare or absent in natural rocky habitats, but very abundant on artificial structures. In Chile, this was apparently the result of predation by fishes and crabs – C. robusta became abundant in rocky habitat when predators were excluded (Dumont et al. 2011).
Food:
Phytoplankton, Bacteria, detritus
Consumers:
fish, crabs
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Marinas & Docks | None |
| General Habitat | Rocky | None |
| General Habitat | Oyster Reef | None |
| General Habitat | Coarse Woody Debris | None |
| General Habitat | Vessel Hull | None |
| Salinity Range | Polyhaline | 18-30 PSU |
| Salinity Range | Euhaline | 30-40 PSU |
| Vertical Habitat | Endobenthic | None |
Tolerances and Life History Parameters
| Minimum Temperature (ºC) | 3 | Field, Venice, average minimum winter temperature (Dybern 1965) |
| Maximum Temperature (ºC) | 30 | Experimental, 36% survival after 15 days, of animals from Venice Lagoon, acclimated at 19 C and 30 ppt (Marin et al. 1987) |
| Minimum Salinity (‰) | 21 | Experimental, adult animals from Venice Lagoon (Marin et al. 1987) |
| Maximum Salinity (‰) | 50 | Experimental, 48% survival after 15 days; animals from Venice Lagoon, acclimated at 19 C and 30 ppt (Marin et al. 1987) |
| Minimum Reproductive Temperature | 12 | Experimental, Venice Lagoon (Marin et al. 1987) |
| Maximum Reproductive Temperature | 28 | Experimental, Venice Lagoon (Marin et al. 1987) |
| Minimum Reproductive Salinity | 29 | Experimental, egg hatching, Venice Lagoon (Marin et al. 1987) |
| Maximum Reproductive Salinity | 50 | Experimental, egg hatching, Venice Lagoon (Marin et al. 1987) |
| Minimum Duration | 0.5 | Egg development time (28 C, 37 PSU, Marin et al. 1987) |
| Maximum Height (mm) | 210 | A specimen from Naples. 100-120 mm is a more typical maximum size (Hoshino and Nishikawa 1985). |
General Impacts
Economic ImpactsShipping and Industry - Ciona robusta and C. intestinalis are widely known as fouling organisms of ships, docks (Visscher 1927; Woods Hole Oceanographic Institution 1951; Millar 1971), aquaculture operations (Rocha et al. 2009), and laboratory seawater systems (Fofonoff, pers. obs.).
Fisheries - The most serious economic impacts of C. robusta have been on shellfish aquaculture in Spain, Chile, Japan, South Africa, and New Zealand (Castilla et al. 2005; Robinson et al. 2005; Rocha et al. 2009). These aquaculture industries are likely affected economically as well. Another negative potential impact of C. robusta and other tunicates is that when they foul aquaculture gear and boats, they can retain and transport viable cells and cysts of toxic phytoplankton (Rosa et al. 2013).
Ecological Impacts
Competition - Ciona robusta is a formidable competitor since it can grow quickly and replace other species in fouling communities, both in its native and introduced ranges (Millar 1971; Lambert and Lambert 2003). Studies in San Francisco Bay, CA have found that it can strongly compete with other native and introduced fouling organisms (Blum et al. 2007). In a similar experiment, near Cape Town, South Africa, removal of C. robusta had no effect on species richness, diversity, or species composition. Impacts of C. robusta can vary with environmental conditions or the composition of the community (Robinson et al. 2017). On settlement panels on the coast of Brittany, C. robusta did not appear to replace or displace C. intestinalis, but was more sensitive to environmental changes, favored by higher temperatures (Bouchemousse et al. 2016). Diversity within fouling communities was negatively correlated with C. robusta abundance, and experimental removal of C. robusta resulted in increased diversity (Blum et al. 2007). Since C. robusta is a strong competitor, fouling of C. robusta on cultured mussels and/or oysters in Spain, South Africa, Chile, Hong Kong, Japan, and New Zealand (Rocha et al. 2009), is likely damaging to shellfish industries. However, effects on wild mussel and other shellfish populations are less clear.
Regional Impacts
| P090 | San Francisco Bay | Ecological Impact | Competition | ||
| In San Francisco Bay, this tunicate appears to strongly compete with other fouling organisms, both native and introduced (Blum et al. 2007). Fouling community diversity was negatively correlated with C. robusta's abundance, and experimental removal of this tunicate resulted in increased diversity (Blum et al. 2007). | |||||
| P090 | San Francisco Bay | Ecological Impact | Habitat Change | ||
| The tunics of C. robusta provided a poor substrate for settlement of other organisms when this organism dominated fouling plates (Blum et al. 2007). | |||||
| NEP-V | Northern California to Mid Channel Islands | Ecological Impact | Competition | ||
| In San Francisco Bay, this tunicate appears to strongly compete with other fouling organisms, both native and introduced (Blum et al. 2007). Fouling community diversity was negatively correlated with C. robusta abundance, and experimental removal of this tunicate resulted in increased diversity (Blum et al. 2007). | |||||
| NEP-V | Northern California to Mid Channel Islands | Ecological Impact | Habitat Change | ||
| The tunics of C. intestinalis provided a poor substrate for settlement of other organisms when this organism dominated fouling plates (Blum et al. 2007). | |||||
| P064 | _CDA_P064 (Ventura) | Ecological Impact | Competition | ||
| Ciona robusta and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| NEP-VI | Pt. Conception to Southern Baja California | Ecological Impact | Competition | ||
| Ciona robusta formed large monospecific patches in San Diego Bay, Mission Bay, Newport Bay, Los Angeles-Long Beach Harbors, King Harbor and Santa Barbara Harbor, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes from southern California harbors (Lambert and Lambert 1998). | |||||
| P062 | _CDA_P062 (Calleguas) | Ecological Impact | Competition | ||
| Ciona robusta and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P065 | _CDA_P065 (Santa Barbara Channel) | Ecological Impact | Competition | ||
| Ciona robusta formed large monospecific patches in Santa Barbara Harbor, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes from southern California harbors (Lambert and Lambert 1998). | |||||
| P060 | Santa Monica Bay | Ecological Impact | Competition | ||
| Ciona robusta formed large monospecific patches in King Harbor, indicating strong competition (Lambert and Lambert 2003). | |||||
| NWP-2 | None | Ecological Impact | Competition | ||
| Fouling of cultured shellfish by C. robusta has been reported in Hong Kong (Huang 2003, cited by da Rocha et al. 2009). | |||||
| NWP-2 | None | Economic Impact | Fisheries | ||
| Fouling of cultured shellfish by C. robusta has been reported in Hong Kong (Huang 2003, cited by da Rocha et al. 2009). | |||||
| P050 | San Pedro Bay | Ecological Impact | Competition | ||
| Ciona robusta formed large monospecific patches in Los Angeles-Long Beach Harbors, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P040 | Newport Bay | Ecological Impact | Competition | ||
| Ciona robusta formed large monospecific patches in Newport Bay, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P027 | _CDA_P027 (Aliso-San Onofre) | Ecological Impact | Competition | ||
| Ciona robusta and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P030 | Mission Bay | Ecological Impact | Competition | ||
| Ciona robusta formed large monospecific patches in Mission Bay, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| P020 | San Diego Bay | Ecological Impact | Competition | ||
| Ciona robusta formed large monospecific patches in San Diego Bay, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| SEP-C | None | Economic Impact | Fisheries | ||
| Ciona robusta has become an abundant fouling organism on cultured scallops in aquaculture operations off Chile (Castillo et al. 2005; Dumont et al. 2011). | |||||
| NWP-3b | None | Economic Impact | Fisheries | ||
| Fouling of cultured shellfish by C. robusta has been reported in Japan (Arakawa, cited by da Rocha et al. 2009). | |||||
| NWP-3b | None | Ecological Impact | Competition | ||
| Fouling of cultured shellfish by C. robusta has been reported in Japan (Arakawa, cited by da Rocha et al. 2009). | |||||
| CA | California | Ecological Impact | Competition | ||
| In San Francisco Bay, this tunicate appears to strongly compete with other fouling organisms, both native and introduced (Blum et al. 2007). Fouling community diversity was negatively correlated with C. robusta abundance, and experimental removal of this tunicate resulted in increased diversity (Blum et al. 2007)., In San Francisco Bay, this tunicate appears to strongly compete with other fouling organisms, both native and introduced (Blum et al. 2007). Fouling community diversity was negatively correlated with C. robusta's abundance, and experimental removal of this tunicate resulted in increased diversity (Blum et al. 2007)., Ciona robusta formed large monospecific patches in Santa Barbara Harbor, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes from southern California harbors (Lambert and Lambert 1998)., Ciona robusta and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., Ciona robusta and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., Ciona robusta formed large monospecific patches in King Harbor, indicating strong competition (Lambert and Lambert 2003)., Ciona robusta formed large monospecific patches in Newport Bay, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., Ciona robusta formed large monospecific patches in Los Angeles-Long Beach Harbors, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., Ciona robusta and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., Ciona robusta formed large monospecific patches in Mission Bay, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998)., Ciona robusta formed large monospecific patches in San Diego Bay, indicating strong competition (Lambert and Lambert 2003). It and other introduced ascidians have replaced the native species Pyura haustor and Ascidia ceratodes in southern California harbors (Lambert and Lambert 1998). | |||||
| CA | California | Ecological Impact | Habitat Change | ||
| The tunics of C. intestinalis provided a poor substrate for settlement of other organisms when this organism dominated fouling plates (Blum et al. 2007)., The tunics of C. robusta provided a poor substrate for settlement of other organisms when this organism dominated fouling plates (Blum et al. 2007). | |||||
Regional Distribution Map
| Bioregion | Region Name | Year | Invasion Status | Population Status | Vectors |
|---|---|---|---|---|---|
| NEP-III | Alaskan panhandle to N. of Puget Sound | 1912 | Non-native | Unknown | |
| NEP-IV | Puget Sound to Northern California | 2000 | Non-native | Unknown | |
| NEP-V | Northern California to Mid Channel Islands | 1932 | Non-native | Established | |
| P112 | _CDA_P112 (Bodega Bay) | 2004 | Non-native | Established | |
| P110 | Tomales Bay | 2001 | Non-native | Established | |
| P090 | San Francisco Bay | 1932 | Non-native | Established | |
| P086 | _CDA_P086 (San Francisco Coastal South) | 2007 | Non-native | Established | |
| P080 | Monterey Bay | 1974 | Non-native | Established | |
| P070 | Morro Bay | 1968 | Non-native | Established | |
| NEP-VI | Pt. Conception to Southern Baja California | 1897 | Non-native | Established | |
| P065 | _CDA_P065 (Santa Barbara Channel) | 1970 | Non-native | Established | |
| P064 | _CDA_P064 (Ventura) | 1994 | Non-native | Established | |
| P062 | _CDA_P062 (Calleguas) | 1994 | Non-native | Established | |
| P060 | Santa Monica Bay | 1970 | Non-native | Established | |
| NEA-III | None | 2003 | Non-native | Established | |
| MED-VII | None | 1875 | Non-native | Established | |
| MED-III | None | 1882 | Non-native | Established | |
| MED-III | None | 0 | Non-native | Established | |
| MED-IV | None | 0 | Non-native | Established | |
| MED-V | None | 1816 | Non-native | Established | |
| NEA-IV | None | 0 | Non-native | Established | |
| MED-II | None | 1884 | Non-native | Established | |
| NWP-3b | None | 0 | Native | Established | |
| NWP-4b | None | 1967 | Native | Established | |
| NWP-3a | None | 0 | Native | Established | |
| MED-VI | None | 0 | Non-native | Established | |
| NWP-2 | None | 1975 | Crypogenic | Established | |
| NWP-4a | None | 0 | Native | Established | |
| P040 | Newport Bay | 1939 | Non-native | Established | |
| WA-IV | None | 1962 | Non-native | Established | |
| WA-V | None | 1955 | Non-native | Established | |
| AUS-VII | None | 1952 | Non-native | Established | |
| NEA-V | None | 2007 | Non-native | Unknown | |
| AUS-VIII | None | 1958 | Non-native | Established | |
| AUS-X | None | 1878 | Non-native | Established | |
| AUS-IX | None | 1952 | Non-native | Established | |
| AUS-V | None | 1928 | Non-native | Established | |
| AUS-IV | None | 1928 | Non-native | Established | |
| NZ-IV | None | 1950 | Non-native | Established | |
| P050 | San Pedro Bay | 1955 | Non-native | Established | |
| P027 | _CDA_P027 (Aliso-San Onofre) | 1996 | Non-native | Established | |
| P023 | _CDA_P023 (San Louis Rey-Escondido) | 2001 | Non-native | Established | |
| P030 | Mission Bay | 1964 | Non-native | Established | |
| P020 | San Diego Bay | 1897 | Non-native | Established | |
| SP-XXI | None | 1933 | Non-native | Established | |
| WA-I | None | 1949 | Non-native | Unknown | |
| WA-VI | None | 1994 | Non-native | Unknown | |
| SA-II | None | 1914 | Non-native | Established | |
| SA-I | None | 1945 | Non-native | Established | |
| SEP-C | None | 2002 | Non-native | Established | |
| SEP-A' | None | 1885 | Non-native | Unknown | |
| RS-3 | None | 2003 | Non-native | Established | |
| MED-I | None | 0 | Non-native | Established | |
| NEA-II | None | 2012 | Non-native | Established | |
| P130 | Humboldt Bay | 0 | Non-native | Failed | |
| RS-1 | None | 2015 | Non-native | Established | |
| EAS-I | None | 1985 | Crypogenic | Unknown | |
| AUS-I | None | 2001 | Non-native | Established | |
| EAS-III | None | 1980 | Crypogenic | Established | |
| AR-IV | None | 2018 | Non-native | Unknown |
Occurrence Map
| OCC_ID | Author | Year | Date | Locality | Status | Latitude | Longitude |
|---|---|---|---|---|---|---|---|
| 767368 | Ruiz et al., 2015 | 2012 | 2012-08-22 | Tomales-Marshall, Bodega Bay, California, USA | Non-native | 38.1514 | -122.8888 |
| 767379 | Ruiz et al., 2015 | 2012 | 2012-08-21 | Tomales-Nick's Cove, Bodega Bay, California, USA | Non-native | 38.1980 | -122.9222 |
| 767399 | Ruiz et al., 2015 | 2012 | 2012-08-16 | Tomales-SNPS, Bodega Bay, California, USA | Non-native | 38.1359 | -122.8719 |
| 767411 | Ruiz et al., 2015 | 2012 | 2012-08-17 | Tomales- Shell Beach, Bodega Bay, California, USA | Non-native | 38.1163 | -122.8713 |
| 767443 | Ruiz et al., 2015 | 2013 | 2013-07-23 | Marina Village, Mission Bay, CA, California, USA | Non-native | 32.7605 | -117.2364 |
| 767460 | Ruiz et al., 2015 | 2013 | 2013-07-29 | Mission Bay Yacht Club, Mission Bay, CA, California, USA | Non-native | 32.7778 | -117.2485 |
| 767480 | Ruiz et al., 2015 | 2013 | 2013-08-04 | Bahia Resort Marina, Mission Bay, CA, California, USA | Non-native | 32.7731 | -117.2478 |
| 767493 | Ruiz et al., 2015 | 2013 | 2013-07-31 | Campland on the Bay, Mission Bay, CA, California, USA | Non-native | 32.7936 | -117.2234 |
| 767511 | Ruiz et al., 2015 | 2013 | 2013-08-01 | Hyatt Resort Marina, Mission Bay, CA, California, USA | Non-native | 32.7634 | -117.2397 |
| 767526 | Ruiz et al., 2015 | 2013 | 2013-08-03 | Mission Bay Sport Center, Mission Bay, CA, California, USA | Non-native | 32.7857 | -117.2495 |
| 767540 | Ruiz et al., 2015 | 2013 | 2013-07-30 | Hilton Resort Docks, Mission Bay, CA, California, USA | Non-native | 32.7791 | -117.2128 |
| 767555 | Ruiz et al., 2015 | 2013 | 2013-08-02 | The Dana Marina, Mission Bay, CA, California, USA | Non-native | 32.7671 | -117.2363 |
| 767566 | Ruiz et al., 2015 | 2013 | 2013-08-05 | Paradise Point Resort, Mission Bay, CA, California, USA | Non-native | 32.7730 | -117.2406 |
| 767668 | Ruiz et al., 2015 | 2013 | 2013-07-16 | Naval Base Point Loma, San Diego Bay, CA, California, USA | Non-native | 32.6886 | -117.2343 |
| 767680 | Ruiz et al., 2015 | 2013 | 2013-07-17 | Naval Station San Diego, San Diego Bay, CA, California, USA | Non-native | 32.6867 | -117.1333 |
| 767694 | Ruiz et al., 2015 | 2013 | 2013-07-24 | NAB ACU-1 Docks, San Diego Bay, CA, California, USA | Non-native | 32.6786 | -117.1615 |
| 767707 | Ruiz et al., 2015 | 2013 | 2013-07-25 | Navy Ammo Dock, Pier Bravo, San Diego Bay, CA, California, USA | Non-native | 32.6939 | -117.2276 |
| 767718 | Ruiz et al., 2015 | 2013 | 2013-07-21 | Cabrillo Isle Marina, San Diego Bay, CA, California, USA | Non-native | 32.7272 | -117.1995 |
| 767746 | Ruiz et al., 2015 | 2013 | 2013-07-18 | NAB Fiddlers Cove, San Diego Bay, CA, California, USA | Non-native | 32.6524 | -117.1486 |
| 767763 | Ruiz et al., 2015 | 2013 | 2013-07-26 | Pier 32 Marina, San Diego Bay, CA, California, USA | Non-native | 32.6516 | -117.1077 |
| 767773 | Ruiz et al., 2015 | 2013 | 2013-07-20 | Chula Vista Marina, San Diego Bay, CA, California, USA | Non-native | 32.6252 | -117.1036 |
| 767786 | Ruiz et al., 2015 | 2013 | 2013-07-28 | Marriott Marquis and Marina, San Diego Bay, CA, California, USA | Non-native | 32.7059 | -117.1655 |
| 767922 | Ruiz et al., 2015 | 2011 | 2011-09-20 | Jack London Square Marina, San Francisco Bay, CA, California, USA | Non-native | 37.7947 | -122.2822 |
| 767988 | Ruiz et al., 2015 | 2012 | 2012-08-24 | Richmond Marina Bay Yacht Harbor, San Francisco Bay, CA, California, USA | Non-native | 37.9134 | -122.3523 |
| 768064 | Ruiz et al., 2015 | 2012 | 2012-09-11 | Ballena Isle Marina, San Francisco Bay, CA, California, USA | Non-native | 37.7676 | -122.2869 |
| 768087 | Ruiz et al., 2015 | 2012 | 2012-08-30 | Oyster Point Marina, San Francisco Bay, CA, California, USA | Non-native | 37.6633 | -122.3817 |
| 768111 | Ruiz et al., 2015 | 2012 | 2012-08-29 | Coyote Point Marina, San Francisco Bay, CA, California, USA | Non-native | 37.5877 | -122.3174 |
| 768133 | Ruiz et al., 2015 | 2012 | 2012-09-04 | Redwood City Marina, San Francisco Bay, CA, California, USA | Non-native | 37.5023 | -122.2130 |
| 768177 | Ruiz et al., 2015 | 2012 | 2012-09-05 | Port of Oakland, San Francisco Bay, CA, California, USA | Non-native | 37.7987 | -122.3228 |
| 768197 | Ruiz et al., 2015 | 2012 | 2012-09-07 | Jack London Square Marina, San Francisco Bay, CA, California, USA | Non-native | 37.7940 | -122.2787 |
| 768253 | Ruiz et al., 2015 | 2012 | 2012-09-12 | Emeryville, San Francisco Bay, CA, California, USA | Non-native | 37.8396 | -122.3133 |
| 768279 | Ruiz et al., 2015 | 2013 | 2013-08-15 | Ballena Isle Marina, San Francisco Bay, CA, California, USA | Non-native | 37.7656 | -122.2858 |
| 768299 | Ruiz et al., 2015 | 2013 | 2013-08-20 | Coyote Point Marina, San Francisco Bay, CA, California, USA | Non-native | 37.5877 | -122.3163 |
| 768318 | Ruiz et al., 2015 | 2013 | 2013-08-22 | Jack London Square Marina, San Francisco Bay, CA, California, USA | Non-native | 37.7926 | -122.2746 |
| 768359 | Ruiz et al., 2015 | 2013 | 2013-08-13 | Oyster Point Marina, San Francisco Bay, CA, California, USA | Non-native | 37.6639 | -122.3821 |
| 768383 | Ruiz et al., 2015 | 2013 | 2013-08-14 | Redwood City Marina, San Francisco Bay, CA, California, USA | Non-native | 37.5024 | -122.2134 |
| 768403 | Ruiz et al., 2015 | 2013 | 2013-08-19 | Richmond Marina Bay Yacht Harbor, San Francisco Bay, CA, California, USA | Non-native | 37.9138 | -122.3522 |
| 768420 | Ruiz et al., 2015 | 2013 | 2013-08-12 | San Francisco Marina, San Francisco Bay, CA, California, USA | Non-native | 37.8078 | -122.4354 |
| 768451 | Ruiz et al., 2015 | 2013 | 2013-08-16 | Sausalito Marine Harbor, San Francisco Bay, CA, California, USA | Non-native | 37.8611 | -122.4851 |
References
Abbott, Donald P.; Newberry, Andrew Todd (1980) Intertidal invertebrates of California, Stanford University Press, Stanford, California. Pp. 177-227Airoldi, Laura; Turon, Xavier; Perkol-Finkel, Shimrit; Rius, Marc (2015) Corridors for aliens but not for natives: effects of marine urban sprawl at a regional scale, Diversity and Distributions 21: 755-768
Asakura, A. (1992) Recent introductions of marine benthos into Tokyo Bay (review): Process of invasion into an urban ecosystem with discussion on the factors inducing their successful introduction, Journal of the Natural History Museum and Institute, Chiba 2(1): 1-14
Astudillo, Juan-Carlos; Wong, Jane C. Y.; Dumont, Clement P.; Bonebrake, Timothy C.; Leung, Kenneth M. Y. (2014) Status of six non-native marine species in the coastal environment of Hong Kong, 30 years after their first record, BioInvasions Records 3(3): in press
Atalaha, Javier; Brook, Rosemary; Cahill, Patrick; Fletcher, Lauren M.; Hopkins, Grant A. (2016) It’s a wrap: encapsulation as a management tool for marine biofouling, Biofouling 32(3): 277-286
Barnard, J. Laurens (1958) Amphipod crustaceans as fouling organisms in Los Angeles-Long Beach Harbors, with reference to the influence of seawater turbidity, California Fish and Game 44(2): 161-170
Barnes, Robert D. (1983) Invertebrate Zoology, Saunders, Philadelphia. Pp. 883
Bastida-Zavala, Rolando J; Ten Hove, Harry A. (2003) Revision of Hydroides Gunnerus, 1758 (Polychaeta: Serpulidae) from the Eastern Pacific region and Hawaii, Beaufortia 53(4): 67-110
Bergström, Per; Thorngren, Linnea; Strand, Lisa; Lindegarth, Mats (2021) Identifying high-density areas of oysters using species distribution modeling: Lessons for conservation of the native Ostrea edulis and management of the invasive Magallana (Crassostrea) gigas in Sweden, Ecology and Evolution Published online: 1-21
Beshai, Ryan A.; Truong, Danny A.; Henry, Amy K. Sorte, Cascade J. B. (2022) Biotic resistance or invasional meltdown? Diversity reduces invasibility but not exotic dominance in southern California epibenthic communities, Biological Invasions 25(2): 533-549
https://doi.org/10.1007/s10530-022-02932-1
bij de Vaate, Abraham ; Hulea, Orieta (2000) Range extension of the Asiatic clam Corbicula fluminea ( Müller 1774) in the River Danube: first record from Romania, Lauterbornia 38: 23-26
Bishop, John D.D.; Wood, Christine A.; Yunnie, Anna L. E.; Griffiths, Carly A. (2015a) Unheralded arrivals: non-native sessile invertebrates in marinas on the English coast, Aquatic Invasions 10: 249-264
Blum, Julia C.; Chang, Andrew L.; Liljesthröm, Marcela; Schenk, Michelle E. Steinberg, Mia K.; Ruiz, Gregory M. (2007) The non-native solitary ascidian Ciona intestinalis depresses species richness., Journal of Experimental Marine Biology and Ecology 342: 5-14
Boffelli, Dario; Weer, Claire V.; Weng, Li; Lewis, Keith D.; Shoukry, Malak I.; Pachter, Lior; Keys, David N.; Rubin, Edward M. (2004) Intraspecies sequence comparisons for annotating genomes, Genome Research 14: 2406-2411
Bouchemousse, Sarah; Bishop, John D. D.; Viard, Frédérique (2016) Contrasting global genetic patterns in two biologically similar, widespread and invasive Ciona species (Tunicata, Ascidiacea), Scientific Reports 6: 24875
Bouchemousse, Sarah; Leveque, Laurent; Dubois, Guillaume; Viard, Frederique (2016) Co-occurrence and reproductive synchrony do not ensure hybridization between an alien tunicate and its interfertile native congener, Evolutionary Ecology 30: 69-87
Boyd, Milton J.; Mulligan, Tim J; Shaughnessy, Frank J. (2002) <missing title>, California Department of Fish and Game, Sacramento. Pp. 1-118
Brewin, Beryl L. (1950) Ascidians of New Zealand Part IV. Ascidians in the vicinity of Christchurch, Transactions of the Royal Society of New Zealand 78(2): 344-353
Brunetti, Riccardo; Gissi, Carmela; Pennati, Roberta; Caicci, Federico; Gasparini, Fabio; Manni, Lucia (2015) Morphological evidence that the molecularly determined Ciona intestinalis type A and type B are different species: Ciona robusta and Ciona intestinalis, Journal of Zoological Systematics and Evolutionary Research Published online: <missing location>
Byrnes, Jarrett; Stachowicz, John J. (2009) Short and long term consequences of increases in exotic species richness on water filtration by marine invertebrates, Ecology Letters 12: 830-841
California Department of Fish and Wildlife (2014) Introduced Aquatic Species in California Bays and Harbors, 2011 Survey, California Department of Fish and Wildlife, Sacramento CA. Pp. 1-36
Caputi, Luigi ; Andreakis, Nikos; Mastrototaro, Francesco; Cirino, Paola; Vassillo, Mauro; Sordino, Paolo (2007) Cryptic speciation in a model invertebrate chordate., Proceedings of the National Academy of Sciences 104(22): 9364-9369
Caputi, Luigi; Crocetta, Fabio; Toscano, Francesco; Sordino, Paolo; Cirino, Paola (2015) Long-term demographic and reproductive trends in Ciona intestinalis sp. A, Marine Ecology 36: 118-128
Carlton, James T. (1979) History, biogeography, and ecology of the introduced marine and estuarine invertebrates of the Pacific Coast of North America., Ph.D. dissertation, University of California, Davis. Pp. 1-904
Carlton, James T. (1979) Introduced invertebrates of San Francisco Bay, In: Conomos, T. J.(Eds.) San Francisco Bay: The Urbanized Estuary. , San Francisco. Pp. 427-444
Carlton, James T.; Eldredge, Lucius (2009) Marine bioinvasions of Hawaii: The introduced and cryptogenic marine and estuarine animals and plants of the Hawaiian archipelago., Bishop Museum Bulletin in Cultural and Environmental Studies 4: 1-202
Castilla, Juan C. and 10 authors (2005) Down under the southeastern Pacific: marine non-indigenous species in Chile., Biological Invasions 7: 213-232
Cavalier-Smith, Thomas (2018) Kingdom Chromista and its eight phyla: a new synthesis emphasising periplastid protein targeting, cytoskeletal and periplastid evolution, and ancient divergences, Protoplasma 255: 297–357
https://link.springer.com/article/10.1007/s00709-017-1147-3
Chang, Andrew Louis (2009) <missing title>, University of California at Davis, Davis CA. Pp. <missing location>
Chavanich, S.; Tan, L. T.; Vallejo, B.; Viyakarn, V. (2010) Report on the current status of marine non-indigenous species in the Western Pacific region, Intergovernmental Oceanographic Commission, Subcommission for the Western Pacific, Bangkok, Thailand. Pp. 1-61
Cheng, Jiawei; Li, Shiguo; Li, Xi; Fu, Ruiying; Huang, Xuena; Aibin, Zhan, (2022) Molecular functional analyses of larval adhesion in a highly fouling invasive model ascidian, Marine Biology 169(120): Published online
https://doi.org/10.1007/s00227-022-04107-x
Chimenz, C.; Fresi, E.; Brunetti, R. (1985) Recherche sui popolamenti di substrato duro de porto d'Ischia: Ascidiacei., Cahiers de Biologie Marine 12: 15-33
Çinar, Melih Ertan and 7 authors (2021) Current status (as of end of 2020) of marine alien species in Turkey, None 16: Published online
Cohen, Andrew N.; Carlton, James T. (1995) Nonindigenous aquatic species in a United States estuary: a case study of the biological invasions of the San Francisco Bay and Delta, U.S. Fish and Wildlife Service and National Sea Grant College Program (Connecticut Sea Grant), Washington DC, Silver Spring MD.. Pp. <missing location>
Cohen, Andrew; and 16 authors. (1998) <missing title>, Washington State Department of Natural Resources, Olympia, Washington. Pp. 1-37
Coles, S. L.; DeFelice, R. C.; Eldredge, L. G.; Carlton, J. T. (1999b) Historical and recent introductions of non-indigenous marine species into Pearl Harbor, Oahu, Hawaiian Islands., Marine Biology 135(1): 147-158
Compton, T. J.; Leathwick, J. R.; Inglis, G. J. (2010) Thermogeography predicts the potential global range of the invasive European green crab (Carcinus maenas), Diversity and Distributions 16: 243-255
Crane, Jules M.; Allen, Larry G.; Eiseman, Connie (1975) Growth rate, distribution, and population density of the Northern Quahog Mercenaria mercenaria in Long Beach, California, California Fish and Game 61(2): 68-81
Cranfield, H.J.; Gordon, D.P.; Willan, R.C.; Marshall, B.A; Battershill, C.N.; Francis, M.P.; Nelson, W.A.; Glasby, C.J.; Read, G.B. (1998) <missing title>, The National Institute of Water and Atmospheric Research, New Zealand. Pp. <missing location>
Currie, D. R.; McArthur, M. A.; Cohen, B. F. (1999) Exotic Marine Pests in the Port of Geelong, Victoria, In: Hewitt, Campbell, Thresher & Martin(Eds.) Marine Biological Invasions of Port Phillip Bay, Victoria. , Hobart, Tasmania. Pp. 227-246
da Rocha, Rosana M.; Bonnet, Nadia Y. K. (2009) [Ascídians (Tunicata, Ascidiacea) introduced to the Alcatraz achipelago, São Paulo], Iheringia Series Zoologie 99: 27-35
da Rocha, Rosana M.; Kremer, Laura P.; Baptista, Mariah S.; Metri, Rafael (2009) Bivalve cultures provide habitat for exotic tunicates in southern Brazil., Aquatic Invasions 4(1): 195-205
de Oliveira Marins, Flávia; da Silva Oliveira, Camila; Macie, Nathalia Maria Vieira; Skinner, Luís Felipe (2009) Reinclusion of Ciona intestinalis (Ascidiacea: Cionidae) in Brazil—a methodological view, JMBA2- Biodiversity Records (online) published online: 1-5
de Rivera, Catherine, and 27 authors (2005) Broad-scale non-indigenous species monitoring along the West Coast in National Marine Sanctuaries and National Estuarine Research Reserves report to National Fish and Wildlife Foundation, National Fish and Wildlife Foundation, Washington, D.C.. Pp. <missing location>
Dias, G. M.; Rocha, R. M.; Lotufo, T. M. C.; Kremer, L. P. (2013) Fifty years of ascidian biodiversity research in Sao Sebastiao, Brazil, Journal of the Marine Biological Association of the United Kingdom 93(1): 273-282
Dumont, C. P.; Gaymer, C. F.; Thiel, M. T (2011) Predation contributes to invasion resistance of benthic communities against the non-indigenous tunicate Ciona intestinalis, Biological Invasions 13: 2023-2034
Dumont, Clément P.; Harris, Larry G.; Gaymer, Carlos F. (2011) Anthropogenic structures as a spatial refuge from predation for the invasive bryozoan Bugula neritina, Marine Ecology Progress Series 427: 95-103
Dybern, Bernt I. (1965) The life cycle of Ciona intestinalis (L.) F. typic in relation to the environmental temperature, Oikos 16: 109-131
Dybern, Bernt I. (1967) The distribution and salinity tolerence of Ciona intestinalis typica with special reference to the waters around souhern scaninavia., Ophelia 4: 207-226
Emara, Ahmed; Belal, Aisha (2004) Marine fouling in Suez Canal, Egypt, Egyptian Journal of Aquatic Research 30A: 189-206
Faasse, Marco A.; Gheerardyn, Hendrik; Morys, Claudia; van Haaren ,Ton; Ysebaer, Tom; Nijland , Reindert (2019) The non-indigenous window shell Theora lubrica Gould, 1861 (Bivalvia: Cardiida: Semelidae) in the delta area of the Netherlands, Basteria 83(1-3): 53-58
Fairey, Russell; Dunn, Roslyn; Sigala, Marco; Oliver, John (2002) Introduced aquatic species in California's coastal waters: Final Report, California Department of Fish and Game, Sacramento. Pp. <missing location>
Fay, R. C.; Vallee, J. A. (1979) A survey of the littoral and sublittoral ascidians of southern California, including the Channel Islands., Bulletin of the Southern California Academy of Sciences 78(2): 122-135
Ferrario, Jasmine; Minchin, Dan (2017) Spread of the non-indigenous serpulid Hydroides sanctaecrucis Krøyer in Mörch, 1863 in the Pacific Ocean: a new record from Taiwan, BioInvasions Records 6(1): 33-38
Foss, Stephen (2009) <missing title>, California Department of Fish and Game, Sacramento CA. Pp. <missing location>
Gauff, Robin P. M.; Lejeusne, Christophe; Arsenieff, Laure ; Bohner, Olivier; Coudret , Jerome; Desbordes, Florian; Jandard, Alise; Loisel, Gaetan (2022) Alien vs. predator: influence of environmental variability and predation on the survival of ascidian recruits of a native and alien species, Biological Invasions Published online: Published online
Green, Stephanie J. and 7 authors (2021) Broad-scale acoustic telemetry reveals long-distance movements and large home ranges for invasive lionfish on Atlantic coral reefs, Marine Ecology Progress Series 673: 117-134
Haderlie, Eugene C.; Donat, Winfield III (1978) Wharf piling fauna and flora in Monterey Harbor, CA, Veliger 21(1): 45-69
Haupt, T. M.; Griffiths, C. L.; Robinson, T. B.;Tonin, A. F. G. (2010) Oysters as vectors of marine aliens, with notes on four introduced species associated with oyster farming in South Africa, African Zoology 45: 52-62
Hewitt, Chad L. and 14 authors. (2004) Introduced and cryptogenic species in Port Phillip Bay, Victoria, Australia., Marine Biology 144: 183-202
Holman, Luke E.; Parker-Nance, Shirley; de Bruyn, Mark; Creer, Simon; Carvalho, Gary; Rius, Marc (2021) Managing human-mediated range shifts: understanding spatial, temporal and genetic variation in marine non-native species, Philosophical Transactions of the Royal Society of London B 377: 20210025
Hoshino, Z.; Nishikawa, T. (1985) Taxonomic Studies of Ciona intestinalis, Publication of the Seto Marine Biological Laboratory 30(1/3): 61-79
Hoshino, Zen'ichiro; Tokioka, Takasi (1967) An unusually robust Ciona from the northeastern coast of Honsyu island, Japan, Publications of the Seto Marine Biological Laboratory 15(4): 275-290
Huisman, John M.; Jones, Diana S.; Wells, Fred E.; Burton, Timothy S. (2008) Introduced marine biota in Western Australian waters., Records of the Western Australian Museum 25: 1-44
Inglis, Graeme ; Gust, Nick; Fitridge, Isla; Floerl, Oliver; Woods, Chris; Hayden, Barbara; Fenwick, Graham (2006f) Port of Napier: Baseline survey for non-indigenous marine species, Biosecurity New Zealand Technical Paper 2005(13): 1-35
Inglis, Graeme and 6 authors (2006c) Port of Nelson: Baseline survey for non-indigenous marine species, Biosecurity New Zealand Technical Paper 2005/02: 1-43
Jacksonville Shell Club The Channeled Applesnail in Northeast Florida. https://www.jaxshells.org/chan.htm
Januario, Stella M.; Estay, Sergio A.; Labra, Fabio A.; Lima, Mauricio (2015) Combining environmental suitability and population abundances to evaluate the invasive potential of the tunicate Ciona intestinalis along the temperate South American coast, PeerJ Published online: <missing location>
Johnston, Emma L.; Keough, Michael J. (2002) Direct and indirect effects of repeated pollution events on marine hard-substrate assemblages, Ecological Applications 12(4): 1212-1228
Karatayev A, Claudi R, Lucy F (2012) History of Dreissena research and the ICAIS gateway to aquatic invasions research, Aquatic Invasions 7(1): 1–5
https://doi.org/10.3391/ai.2012.7.1.001
Karatayev AY, Burlakova LE, Karatayev VA, Boltovskoy D (2010) Limnoperna fortunei versus Dreissena polymorpha: population densities and benthic community impacts of two invasive freshwater bivalves, Journal of Shellfish Research 29(4): 975–984
https://doi.org/10.2983/0730-8000\(2007\)26[205:TIBDPA]2.0.CO;2
Keough, M. J. ; Ross, J. (1999) Introduced fouling species in Port Phillip Bay., In: Hewitt, C. L.; Campbell; M.;Thresher, R.; Martin,(Eds.) Marine Biological Invasions of Port Phillip Bay, Victoria. , Hobart, Tasmania. Pp. 193-225
Kott, P. (1998) Tunicata, Zoological Catalogue of Australia 34: 51-252
Kott, P. (2005) Catalogue of Tunicata in Australian waters, Queensland Museum, Brisbane. Pp. 1-301
Kott, Patricia (1990) The Australian Ascidiacea, part 2, Aplousobranchia (1), Memoirs of the Queensland Museum 29(1): 1-266
Koukouras, Athanasios; Voultisiado-Koukoura, Eleni; Kevrekidis, Theodoros; Vafidis, Dimitri (1995) Ascidian fauna of the Aegean Sea with a checklist of the Mediterranean and Black Sea species, Annales de l Institut Oceanographique, Paris 71(1): 19-34
Lambert, C. C.; Lambert, G. (1998) Non-indigenous ascidians in southern California harbors and marinas., Marine Biology 130: 675-688
Lambert, Charles C; Lambert, Gretchen (2003) Persistence and differential distribution of nonindigenous ascidians in harbors of the Southern California Bight., Marine Ecology Progress Series 259: 145-161
Lambert, Gretchen (2003) New records of ascidians from the NE Pacific: a new species of Tridemnum, range extension and description of Aplidiopsis pannosum (Ritter, 1899), including its larva, and several non-indigenous species., Zoosystema 24(4): 665-675
Lee, Taekjun; Shin, Sook (2014) Morphological and molecular identification of an introduced alien sea squirt (Tunicata: Ascidiacea) in Korea, Proceedings of the Biological Society of Washington 127(1): 284-297
Lin, Yaping; Chen, Yiyong; Xiong, Wei; Zhan, Aibin (2016) Genomewide gene-associated microsatellite markers for the model invasive ascidian, Ciona intestinalis species complex, Molecular Ecology Resources 16: 784-793
Lopes, Rubens M. (Ed.) (2009) <missing title>, Ministry of the Environment, Brasilia, Brazil. Pp. 1-440
Low-Pfeng, Antonio; Recagno, Edward M. Peters (2012) <missing title>, Geomare, A. C., INESEMARNAT, Mexico. Pp. 236
MacGinitie, G. E.; MacGinitie, Nettie (1968) <missing title>, McGraw-Hill, New York NY. Pp. <missing location>
Madariaga, David Jofre; Rivadeneira, Marcelo M.; Tala, Fadia; Thiel, Martin (2014) Environmental tolerance of the two invasive species Ciona intestinalis and Codium fragile: their invasion potential along a temperate coast, Biological Invasions 16(12): 2507-2527
Marin, M. G.; Bressan, M; Beghi, L.; Brunetti, R. (1987) Thermo-haline tolerance of Ciona intestinalis,/i> at different development stages., Cahiers de Biologie Marine 28: 47-57
McDonald, J. (2004) The invasive pest species Ciona intestinalis reported in a harbour in southern western Australia., Marine Pollution Bulletin 49: 854-874
McDonald, J. I.; Wilkens, S. L.; Stanley, J. A.; Jeffs, A. G. (2014) Vessel generator noise as a settlement cue for marine biofouling species, Biofouling 30(6): 741-749
McLaughlin, Janelle; Bourque, Daniel; LeBlanc, Angeline R.; Fortin, Guillaume (2013) Effect of suspended inorganic matter on fertilization success, embryonic development, larval settlement, and juvenile survival of the vase tunicate Ciona intestinalis (Linnaeus, 1767), Aquatic Invasions 8(4): 375-388
Mead, A.; Carlton, J. T.; Griffiths, C. L.; Rius, M. (2011a) Revealing the scale of marine bioinvasions in developing regions: a South African re-assessment, Biological Invasions 13(9): 1991-2008
Mead, A.; Carlton, J. T.; Griffiths, C. L. Rius, M. (2011b) Introduced and cryptogenic marine and estuarine species of South Africa, Journal of Natural History 39-40: 2463-2524
Millar, R. H. (1958) Some ascidians from Brazil., Annals and Magazine of Natural History 13(1): 497-514
Millar, R.H. (1971) Ascidians as fouling organisms., In: Jones, E.B. Gareth; Eltringham S.K.(Eds.) Marine borers, fungi and fouling organisms of wood. , Brussels. Pp. 185-195
Monniot, Claude; Monniot, Francoise (1994) Additions to the inventory of Eastern tropical Atlantic Ascidians: arrival of cosmopolitan species., Bulletin of Marine Science 54(1): 71-93
Monniot, Claude; Monniot, Francoise; Griffiths, Charles; Schleyer, Michael (2001) South African Ascidians., Annals of the South African Museum 108(1): 1-141
Morton, Brian (1987) Recent marine introductions into Hong Kong, Bulletin of Marine Science 41(2): 503-513
Moura, Carlos J.; Collins, Allen G.; Santos, Ricardo S.; Lessios, Harilaos (2019) Predominant east to west colonizations across major oceanic barriers: Insights into the phylogeographic history of the hydroid superfamily Plumularioidea, suggested by a mitochondrial DNA barcoding marker, Ecology and Evolution 9: :13001–13016.
DOI: 10.1002/ece3.5608
Naranjo, S. A.; Carballo, J. C.; Garcia-Gomez, J. C. (1996) Effects of environmental stress on ascidian populations in Algeciras Bay (southern Spain)., Marine Ecology Progress Series 144: 119-131
Needles, Lisa A. (2007) <missing title>, M.S. Thesis, California Polytechnic State University, San Luis Obispo. Pp. <missing location>
Needles, Lisa A.; Wendt, Dean E. (2013) Big changes to a small bay: Introduced species and long-term compositional shifts to the fouling community of Morro Bay (CA), Biological Invasions 15(6): 1231-1251
Nichols, Claire L.; Lambert, Gretchen; Nydam, Marie L. (2023) Continued persistence of non-native ascidians in Southern California harbors and marinas, Aquatic Invasions 18(1): 1-22
https://doi.org/10.3391/ ai.2023.18.1.101962
Nishikawa, T. (1991) The ascidians of the Japan Sea. II., Publications of the Seto Marine Biological Laboratory 35: 25-170
Nishikawa, Teruaki (1992) The Ascidians of the Japan Sea III., Publications of the Seto Marine Biological Laboratory 35(6): 303-334
Nishikawa, Teruki (1991) The Ascidians of the Japan Sea., Publication of the Seto Marine Biological Laboratory 35(1/3): 25-170
Nydam, Marie L.; Harrison, Richard G. (2007) Genealogical relationships within and among shallow-water Ciona species (Ascidiacea)., Marine Biology 151: 1839-1847
Nydam, Marie L.; Harrison, Richard G. (2010) Polymorphism and divergence within the ascidian genus Ciona, Molecular Phylogenetics and Evolution 56: 718-726
Nydam, Marie L.; Harrison, Richard G. (2011) Introgression despite substantial divergence in a broadcast spawning marine invertebrate, Evolution 65(3): 429-442
Occhipinti-Ambrogi, Anna; Galil, Bella S. (2004) A uniform terminology on bioinvasions: a chimera or an operative tool?, Marine Pollution Bulletin 49: 688-694
Orensanz, Jose Maria and 14 other authors (2002) No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic, Biological Invasions 4(1-2): 115-143
Pante, Eric and 11 authors (2015) Species are hypotheses: avoid connectivity assessments based on pillars of sand, Molecular Ecology 34: 525-544
Pascoe, P. L.; Parry, H. E.; Hawkins, A. J. S. (2007) Dynamic filter-feeding responses in fouling organisms, Aquatic Biology 1: 177-185
Pennati, Roberta and 10 authors (2015) Morphological differences between larvae of the Ciona intestinalis species complex: hints for a valid taxonomic definition of distinct species, None 10: e.0122879
Pestana, Lueji Barros; Dias, Gustavo Muniz; Marquesa, Antonio Carlos (2017) A century of introductions by coastal sessile marine invertebrates in Angola, South East Atlantic Ocean, Marine Pollution Bulletin 125: 426-a432
Puntila-Dodd, R.; . Bekkevold, D.; .Behrens, J. W. (2021) Estimating salinity stress via hsp70 expression in the invasive round goby (Neogobius melanostomus): implications for further range expansion, Hydrobiologia 848: 421–429
Pyo, Jooyeon; Lee, Taekjun; Shin, Sook (2012) Two newly recorded invasive alien ascidians (Chordata, Tunicata, Ascidiacea) based on morphological and molecular phylogenetic analysis in Korea, Zootaxa 3368: 211-228
Rho, Boon Jo (1995) The Ascidians (Tunicata) from Chindo Islands, Korea, Korean Journal of Systematic Zoology 11(1): 125-145
Rho, Boon Jo; Lee, Ji-Eun (1991) A systematic study of the Ascidians in Korea, Korean Journal of Systematic Zoology 7(2): 195-220
Rho, Boon Jo; Park, Kyung-Sook (1998) Taxonomy of ascidians from Geojedo Island in Korea, Korean Journal of Systematic Zoology 14(3): 173-192
Rius, M.; Griffths, C. W. (2011) Alien & Invasive Animals: A South African Perspective, Random House Struik, Johannesburg, South Africa. Pp. 71-75
Rius, Marc and 7 authors (2014) Range expansions across ecoregions: interactions of climate change, physiology and genetic diversity, Diversity and Distributions 23: 76-88
Rius, Marc; Branch, George M.; Griffiths, Charles L.; Turon, Xavier (2010) Larval settlement behaviour in six gregarious ascidians in relation to adult distribution, Marine Ecology Progress Series 418: 151-163
Rius, Marc; Potter, Elaine E.; Aguirre, J. David; Stachowicz, John J. (2014) Mechanisms of biotic resistance across complex life cycles, Journal of Animal Ecology 83: 296-305
Robinson, T. B.; Griffiths, C. L.; McQuaid, C. D.; Rius, M. (2005) Marine alien species of South Africa-- status and impacts, African Journal of Marine Science 27(1): 297-306
Rodriguez, Laura F.; Ibarra-Obando, Silvia E. (2008) Cover and colonization of commercial oyster (Crassostrea gigas) shells by fouling organisms in San Quintin Bay, Mexico, Journal of Shellfish Research 27(2): 337-343
Rosa, M. and 6 authors (2013) Biofouling ascidians on aquaculture gear as potential vectors of harmful algal introductions, Harmful Algae 23: 1-7
Roux, Camille; Tsagkogeorga, Georgia; Bierne, Nicolas; Galtier, Nicolas (2013) Crossing the species barrier: Genomic hotspots of introgression between two highly divergent Ciona intestinalis species, Molecular Biology and Evolution 30(7): 1574-1587
Ruiz, Gregory M.; Geller, Jonathan (2018) Spatial and temporal analysis of marine invasions in California, Part II: Humboldt Bay, Marina del Re, Port Hueneme, and San Francisco Bay, Smithsonian Environmental Research Center & Moss Landing Laboratories, Edgewater MD, Moss Landing CA. Pp. <missing location>
Ruiz, Gregory; Geller, Jonathan (2021) Spatial and temporal analysis of marine invasions: supplemental studies to evaluate detection through quantitative and molecular methodologies, Marine Invasive Species Program, California Department of Fish and Wildlife, Sacramento CA. Pp. 153 ppl.
Sanamyan, Karen; Schories, Dirk (2005) Ascidians from Peru, Spixiana 27(3): 193-197
Sato, Atsuko; Satoh, Nori; Bishop, John D. D. (2012) Field identification of ‘types’ A and B of the ascidian Ciona intestinalis in a region of sympatry, Marine Biology 159: 1611-1619
Schwindt, Evangelina and 15 authors (2014) Marine fouling invasions in ports of Patagonia (Argentina) with implications for legislation and monitoring programs, Marine Environmental Research 99: 60-68
Silva, Nathan; Smith, William C. (2008) Inverse correlation of population similarity and introduction date for invasive ascidians, None 3(6): available online
Simkanin, Christina; Fofonoff, Paul W.; Larson, Kriste; Lambert, Gretchen; Dijkstra, Jennifer A.; Ruiz, Gregory M. (2016) Spatial and temporal dynamics of ascidian invasions in the continental United States and Alaska, Marine Biology 163: Published online
Smale, Dan A.; Wernberg, Thomas (2012) Short-term in situ warming influences early development of sessile assemblages, Marine Ecology Progress Series 453: 129-136
Smith, Kirsty F.; Cahill, Patrick L.; Fidler, Andrew E. (2010) First record of the solitary ascidian Ciona savignyi Herdman, 1882 in the Southern Hemisphere, Aquatic Invasions 5(4): 363-368
Stachowicz, John J.; Byrnes, Jarrett E. (2006) Species diversity, invasion success, and ecosystem functioning: disentangling the influence of resource competition, facilitation, and extrinsic factors., Marine Ecology Progress Series 311: 251-262
Suzuki, Miho M.; Nishikawa, Teruaki ; Bird, Adrian (2005) Genomic approaches reveal unexpected genetic divergence within Ciona intestinalis., Journal of Molecular Evolution 61: 627-635
Thorarinsdottir, Gudrun G.; Gunnarsson, Karl; Gíslason, Ó. Sindri (2014) Marine invasive species in the Arctic, Nordic Council of Ministers, Copenhagen, Denmark. Pp. 83-109
Tracy, Brianna M.; Reyns, Nathalie B. (2014) Spatial and temporal patterns of native and invasive ascidian assemblages in a Southern California embayment, Aquatic Invasions 9: In press
Turon, Xavier; Cañete, Juan I.; Sellanes, Javier; Rocha, Rosana M.; López-Legentil, Susanna (2016) Too cold for invasions? Contrasting patterns of native and introduced ascidians in subantarctic and temperate Chile, Management of Biological Invasions 7: 77-86
U.S. National Museum of Natural History 2002-2021 Invertebrate Zoology Collections Database. http://collections.nmnh.si.edu/search/iz/
Valdivia, Nelson; Heidemann, Astrid; Thiel, Martin; Molis, Markus, Wahl, Martin (2005) Effects of disturbance on the diversity of hard-bottom macrobenthic communities on the coast of Chile., Marine Ecology Progress Series 299: 45-54
Van Name, Willard G. (1912) Simple ascidians of the coasts of New England and neighboring British provinces., Proceedings of the Boston Society of Natural History 34: 439-619
Van Name, Willard G. (1945) The North and South American ascidians, Bulletin of the American Museum of Natural History 84: 1-462
Vazquez, E.; Urgorri, V. (1992) [Fouling ascidians in the inlet of A Grana, Ria de Ferrol, (Galicia, Spain)], Nova Acta Cientifica Compostelana (Bioloxia) 3: 161-167
Wilson, Emily Erin (2011) <missing title>, Humboldt State University, Eureka CA. Pp. <missing location>
Wiltshire, K.; Rowling, K.; Deveney, M. (2010) <missing title>, South Australian Research and Development Institute, Adelaide. Pp. 1-232
Woods Hole Oceanographic Institution, United States Navy Dept. Bureau of Ships (1952) Marine fouling and its prevention., United States Naval Institute., Washington, D.C.. Pp. 165-206
Zhan, Aibin; Briski, Elizabeta; Bock, Dan G.; Ghabooli, Sara; MacIsaac, Hugh J. (2015) Ascidians as models for studying invasion success, Marine Biology 162: 2449-2470
Zhan, Aibin; MacIsaac, Hugh J .; Cristescu, Melania E. (2010) Invasion genetics of the Ciona intestinalis species complex: from regional endemism to global homogeneity complex: from regional endemism to global homogeneity, Molecular Ecology 19: 4678-4694