Invasion History
First Non-native North American Tidal Record:First Non-native West Coast Tidal Record:
First Non-native East/Gulf Coast Tidal Record:
General Invasion History:
None
North American Invasion History:
Description
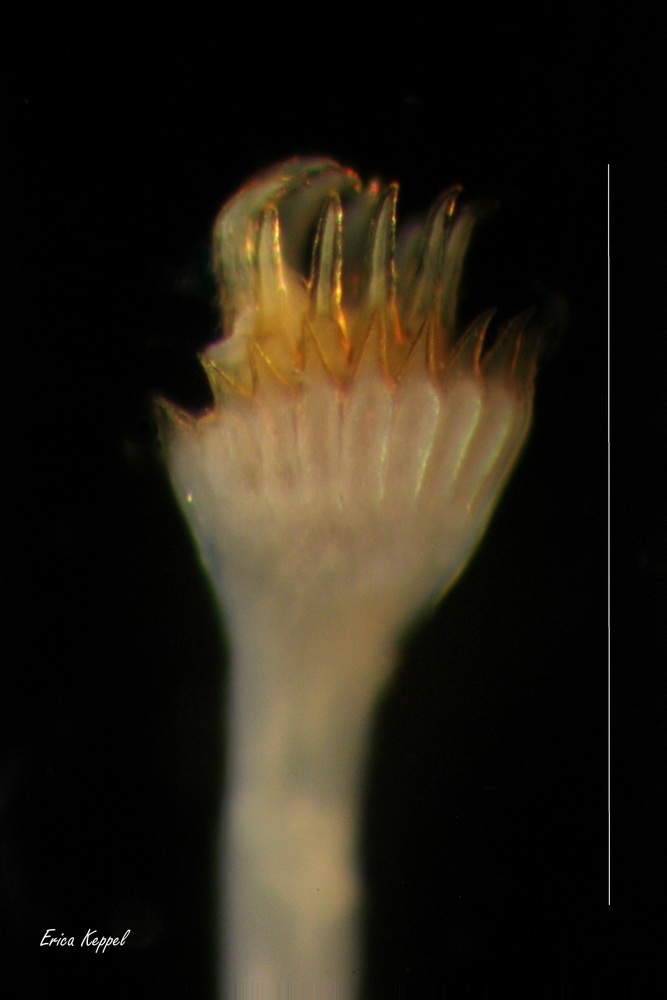
Hydroides dianthus secretes a calcareous tube, as do other serpulid polychaetes. Serpulids have a feathery crown of modified prostomial palps, called radioles (the prostomium is the first segment, projecting above the mouth). The radioles can be folded and withdrawn into the tube. One of tne of the radioles is modified to form an operculum, which acts as a plug when the animal contracts. The peristomium (segment behind the mouth) is folded back to form a collar, which bears uniramous parapodia, with a distinctive set of collar chaetae, with spines or serrations. The collar is the first of seven thoracic chaeta-bearing segments (chaetigers). The subsequent segments have biramous parapodia. The dorsal branch of the parapodium is called the notopodium; the ventral isne the neuropodium. Chaetae in the two branches and along the body can vary greatly in their morphology, which can be critical in the taxon. The tubes have two longitudinal ridges, and lack a peristome (flared opening). The branchial (gill) crown consists of about 16 radioles each on the left and right sides of the mouth. It comprises about 1/4 of the worm's length.e.. The peduncle is cylindrical. The opercular funnel has 31 radii with pointed tips. The verticil has 10 yellowish spines, all bending ventrally. The verticil lacks a central tooth. The collar chaetae:are bayonet chaetae, with two blunt-rounded to short teeth at their base, and a smooth distal blade. The thorax has six chaetigers bearing short, rasp-like setae, called uncinae, and limbate chaetae. The abdomen has about 89 segments (69-119, n=4). The overall length is about 16.5 mm (8-30, n=54. The worm is yellow to light brown. (Description from Bastida-Zavala and ten Hove 2002; Bastida-Zavala 2017).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Annelida | |
| Class: | Polychaeta | |
| Subclass: | Palpata | |
| Order: | Canalipalpata | |
| Suborder: | Sabellida | |
| Family: | Serpulidae | |
| SubFamily: | Serpulinae | |
| Genus: | Hydroides | |
| Species: | dianthus |
Synonyms
Hydroides dianthoides (Augener, 1922)
Serpula dianthus (Verrill, 1873)
Serpula dianthus var. citrina (Verrill, 1873)
Potentially Misidentified Species
None
Hydroides elegans
None
Hydroides gairacensis
None
Hydroides santacrucis
None
Ecology
General:
Life History – The serpulid polychaete Hydroides dianthus feeds by extending its feathery gills and trapping plankton in the water column, which are transported by cilia to the mouth. The sexes are separate, as in most serpulid species. The larvae are planktotrophic and spend about 4-16 days in the plankton at 21-⁰C, with development being sdelayed at low food levels (Toonen and Pawli 2001). Temperature doubtless affects larval development, but we have noit found references.
Ecology – Hydroides dianthus tolerates salinities as low as 1 PSU , but is usually associated with marine salinities (30-37 PSU, Bastida-Zavala and ten Hove 2002). However, in the Suez Canal, it was found in the Great Bitter Lakes and Lake Timsah, where salinities exceeded 40 PSU. To our knowledge, the temperature tolerance of H. elegans has not been studied experimentally. Hydroides elegans is comparatively tolerant of tributyltin antifouling compounds, wood preservative chemicals, low oxygen, and hydrogen sulfide, and benefits from the dense phytoplankton concentrations in polluted harbors (Udhayakumar and Karande 1996; Tarakanadha et al. 2004). It secretes a calcareous tube, often irregularly coiled, on hard surfaces such as rocks, pilings, floats, shells, corals, mangroves, and ships’ hulls. While it often forms dense aggregations on surfaces, it is not known to form reefs. The aggregations of worms appear to result from hydrodynamic processes and passive settlement, rather than by chemical cues and active swimming (Walters et al. 1999).
Food:
Phytoplankton
Trophic Status:
Suspension Feeder
SusFedHabitats
Tolerances and Life History Parameters
| Minimum Salinity (‰) | 1 | Filed (Bastida-Zavala and ten Hove 2017) |
| Maximum Salinity (‰) | 51.7 | Filed (Bastida-Zavala and ten Hove 2017) |
| Minimum Duration | 4 | Toonen and Pawlik 2001 (experimental, 21 C, fed ) |
| Maximum Duration | 18 | Toonen and Pawlik 2001 (experimental, 21 C, starved) |
| Minimum Length (mm) | 8 | Bastida-Zavala and ten Hove 2002 |
| Maximum Length (mm) | 30 | Bastida-Zavala and ten Hove 2002 |
| Broad Temperature Range | None | Cold temperate-Subtropical |
| Broad Salinity Range | None | Oligohaline-Hyprehaline |
General Impacts
Regional Impacts
| MED-III | None | Ecological Impact | Habitat Change | ||
| Construction of reefs | |||||
| NWP-4a | None | Ecological Impact | Habitat Change | ||
| cboIMP_Impact txtIMP_Type IMP_Comments Habitat Change Ecological Impact Hydroides dianthus reefs provided habitat for dense populations of polyps of the native jellyfish Aurelia coeruea (Dong et al. 2018). | |||||
| NWP-3a | None | Ecological Impact | Habitat Change | ||
| Reefs formed by H. dianthus provided habitat for dense aggregations of polyps of Aurelia coerulea. | |||||
Regional Distribution Map
| Bioregion | Region Name | Year | Invasion Status | Population Status | Vectors |
|---|---|---|---|---|---|
| NA-ET3 | Cape Cod to Cape Hatteras | 1873 | Crypogenic | Established | |
| CAR-VII | Cape Hatteras to Mid-East Florida | 0 | Crypogenic | Established | |
| MED-II | None | 1967 | Crypogenic | Established | |
| CAR-I | Northern Yucatan, Gulf of Mexico, Florida Straits, to Middle Eastern Florida | 0 | Crypogenic | Established | |
| MED-III | None | 1888 | Crypogenic | Established | |
| NWP-3b | None | 2006 | Non-native | Established | |
| NEA-II | None | 1970 | Non-native | Established | |
| MED-V | None | 0 | Non-native | Established | |
| MED-VII | None | 1874 | Crypogenic | Established | |
| MED-IV | None | 0 | Non-native | Established | |
| MED-VI | None | 1861 | Crypogenic | Established | |
| NEA-V | None | 1927 | Non-native | Established | |
| NEA-IV | None | 0 | Non-native | Established | |
| CAR-II | None | 0 | Crypogenic | Established | |
| SEP-H | None | 2008 | Non-native | Established | |
| WA-I | None | 0 | Non-native | Established | |
| NWP-4a | None | 2013 | Non-native | Established | |
| MED-IX | None | 2009 | Non-native | Established | |
| PAN_PAC | Panama Pacific Coast | 2008 | Non-native | Established | |
| SA-II | None | 2012 | Non-native | Established | |
| NWP-3a | None | 2016 | Non-native | Established | |
| NA-ET2 | Bay of Fundy to Cape Cod | 1975 | Crypogenic | Established |
Occurrence Map
| OCC_ID | Author | Year | Date | Locality | Status | Latitude | Longitude |
|---|
References
Aviz, Daiane; Da Silva, Roseanne Figueira; Filho, José Souto (2018) Sabellaria wilsoni (Polychaeta: Sabellariidae): an ecosystem engineer and promoter of zoobenthos diversity in the Brazilian Amazon coast, Journal of the Marine Biological Association of the United Kingdom 99(5): 1099-1109DOI: https://doi.org/10.1017/S0025315418001157
Bastida-Zavala, J. Rolando; McCann, Linda D.; Keppel; Erica; Ruiz, Gregory M. (2017) The fouling serpulids (Polychaeta: Serpulidae) from United States coastal waters: an overview, European Journal of Taxonomy 344: 1-76
Shalovenko, N. N. (2020) Tendencies of Invasion of Alien Zoobenthic Species into the Black Sea, Russian Journal of Biological Invasions 11(2): . 164–171
Abdelsalam, Khaled Mahmood (2018) First record of the exotic lysmatid shrimp Lysmata vittata (Stimpson, 1860) (Decapoda: Caridea: Lysmatidae) from the Egyptian Mediterranean coast, Mediterranean Marine Science 19(1): 124-131
Bastida-Zavala, J. Rolando; Ten Hove, Harry A. (2002) Revision of Hydroides Gunnerus, 1768 (Polychaeta: Serpulidae) from the Western Atlantic region., Beaufortia 52(9): 103-178
Ben-Eliahu, M. N. (1976) Polychaeta cryptofauna from rims of similar intertidal vermetid reefs on the Mediterranean coast of Israel.and in the Gulf of Elat: Serpulidae (Polychaeta Sedentaria), Israel Journal of Zoology 25: 103-119
Ben-Eliahu, M. Nechama; ten Hove, Harry A. (2011) Serpulidae (Annelida: Polychaeta) from the Suez Canal: From a Lessepsian migration perspective (a monograph), Zootaxa 2848: 1-147
Bianchi, C. N.; Morri, C. (2001) The battle is not to the strong: Serpulid reefs in the lagoon of Orbetello (Tuscany: Italy)., Estuarine, Coastal and Shelf Science 53: 215:220
Blakeslee, April M. H.; Miller, A. Whitman; Ruiz, Gregory M.; Kerstin Johannesson · Carl André ·Johannsson, Kerstin;· André, Carl; Panova, Marina (2021) Population structure and phylogeography of two North Atlantic Littorina species with contrasting larval development, Marine Biology 168: <missing location>
Çinar, Melih Ertan (2013) Alien polychaete species worldwide: current status and their impacts, Journal of the Marine Biological Association of the United Kingdom 93(5): 1257-1278
Cornelio, Michele; Manzoni, Alberto (1999) Caratterizzazione stagionale degli insediamenti di organismi macrobentonici su substrati sperimentali nel bacino centrale della laguna di Venezia., Bollettino del Museo Civico di Storia Naturale di Venezia 49: 135-144
Dong, Zhijun; Sun, Tingting; Wang, Lei (2018) The biogenic reefs formed by the alien polychaete Hydroides dianthus (Serpulidae, Annelida) favor the polyp stage of Aurelia coerulea (Cnidaria, Scyphozoa) in a coastal artificial lake, Marine Pollution Bulletin <missing volume>: 86-91
Ghobashy, Abdel Fattah A.; Ghobashy, Mahi A. F. (2005) Marine fouling studies in Egypt: A- Serpulids, Egyptian Journal of Aquatic Research 31(2): 89-102
Goulletquer, Philippe; Bachelet, Guy; Sauriau, Pierre; Noel, Pierre (2002) Invasive aquatic species of Europe: Distribution, impacts, and management, Kluwer Academic Publishers, Dordrecht. Pp. 276-290
Hall, Stephanie A.; Stewart-Clark, Sarah E.; Kupriyanov, Elena (2022) Spatial and temporal monitoring of invasive Hydroides dianthus (Verrill, 1873) (Annelida, Serpulidae) in Eel Lake, Argyle, Nova Scotia using a species-specific molecular assay, Management of Biological Invasions 13: Published online
Karatayev AY, Burlakova LE, Karatayev VA, Boltovskoy D (2010) Limnoperna fortunei versus Dreissena polymorpha: population densities and benthic community impacts of two invasive freshwater bivalves, Journal of Shellfish Research 29(4): 975–984
https://doi.org/10.2983/0730-8000\(2007\)26[205:TIBDPA]2.0.CO;2
Knight-Jones, Phyllis; Knight-Jones, Wyn; Ergen, Zeki (1991) Sabelliform polychaetes, mostly from Turkey's Aegean coast, Journal of Natural History 25(4): 837-858
Leone, Donald E. (1970) The maturation of Hydroides dianthus, Biological Bulletin 138(3): 306-315
Link, Heike; Nishi, Ejiroh; Tanaka, Katsuhiko; Bastida-Zavala, Rolando; Kupriyanova, Elena K.; Yamakita, Takehisa (2009) Hydroides dianthus (Polychaeta: Serpulidae), an alien species introduced into Tokyo Bay, Japan., Marine Biodiversity Records <missing volume>: 1-6
Otani, Michio; Yamanishi, Ryohei (2010) Distribution of the alien species Hydroides dianthus (Verrill, 1873) (Polychaeta: Serpulidae) in Osaka Bay, Japan, with comments on the factors limiting its invasi, Planton and Benthios Research 5(2): 62-68
Pancuci-Papadopoulou., M. A.; Zenetos, A , Corsini-Foka; Politou, Ch. (2005) Update of marine alien species in Hellenic waters., Mediterranean Marine Science 6(2): 1-11
Pederson, Judith, and 13 authors (2021) 2019 Rapid Assessment Survey of marine bioinvasions of southern New England and New York, USA, with an overview of new records and range expansions, Bioinvasions Records 10(2): 22-–237
Qiu, J.W.; Qian, P-Y (1997) Combined effects of salinity, temperature and food on early development of the polychaete Hydroides elegans., Marine Ecology Progress Series 152: 79-88
Robinson, C. B. (1904) The distribution of Fucus serratus in North America, Torreya 3: 132-134
Rodrigues, Andrielle Raposo; Skinner, Luis Felipe; dos Santos Brasil, Ana Claudia (2021) Do Morphological Similarities and human-induced dispersal explain the non-native occurrence of Serpulidae (Annelida) in Southwest Atlantic? Taxonomic detailing is the key, Papeis Avulsos Zoologia de Sao Paolo 60: Published online
Toonen, Robert J.; Pawlik, Joseph R. (2001) Settlement of the gregarious tube worm Hydroides dianthus (Polychaeta: Serpulidae). II. Testing the desperate larva hypothesis, Marine Ecology Progress Series 224: 115-131
U.S. National Museum of Natural History 2002-2021 Invertebrate Zoology Collections Database. http://collections.nmnh.si.edu/search/iz/
Verrill, A.E.; Smith, S.I. (1873) <missing title>, 1 Report of the United States Commission of Fish and Fisheries, <missing place>. Pp. 1-757
Wang, Jianjun; Huang, Zongguo, (1993) Fouling polychaetes of Hong Kong and adjacent waters., Asian Marine Biology 10: 1-12
Wong, Eunice; Kupriyanova, Elena K.; Hutchings, Pat; Capa, María; Radashevsky, Vasily; ten Hove, Harry A. (2014) A graphically illustrated glossary of polychaete terminology: invasive species of Sabellidae, Serpulidae and Spionidae, Memoirs of Museum of Victoria 71: 327-342
Woods Hole Oceanographic Institution, United States Navy Dept. Bureau of Ships (1952) Marine fouling and its prevention., United States Naval Institute., Washington, D.C.. Pp. 165-206
Zibrowius, Helmut (1981) Serpulidae (Anellida, Polychaeta) indopacifiques etablis dans le region de Beyrouth, Liban., Rapportes de la Commision Internationale de la Mer Mediterranee 27(2): 159-160
Zibrowius, Helmut (1991) Ongoing modification of the Mediterranean marine fauna and flora by the establishment of exotic species., Mesogee 51: 83-107
Zibrowius, Helmut (1994) Introduced Species in European Coastal Waters, European Commission, Brussels. Pp. 44-65
Zibrowius, Helmut, Thorp, Clifford H. (1989) A review of the alien serpulid and spirorbid polychaetes in the British Isles, Cahiers de Biologie Marine 30(3): 271-285