Invasion History
First Non-native North American Tidal Record: 1913First Non-native West Coast Tidal Record: 1913
First Non-native East/Gulf Coast Tidal Record:
General Invasion History:
Eurytemora carolleeae was recently separated from the circumboreal E. affinis species complex (Lee 2000; Alekseev & Souissi 2011). Lee (2000), using mitochondrial RNA and genomic DNA, divided Eurytemora 'affinis' into a number of clades and subclades. Several of the subclades of Eurytemora ‘affinis’ occur in very close proximity, but appear to be reproductively isolated. The ‘Atlantic’ subclade, subsequently named E. carolleeae, ranges from salt marshes in the St. Lawrence River, Canada to the St. Johns River, Florida (Lee 2000; Alekseev & Souissi 2011). Genetic analysis indicates that this species has been introduced to at least two estuaries on the Pacific coast of North America and to the Great Lakes (Lee 2000), and also to the Gulf of Finland, Baltic Sea (Alekseev et al. 2009). This copepod is euryhaline and capable of surviving and reproducing in both fresh and marine waters (Lee 2000).
North American Invasion History:
Invasion History on the West Coast:
Lee's (1999; 2000) analysis detected Eurytemora carolleeae in San Francisco Bay and in a salt marsh near Grays Harbor, Washington. Other Pacific Coast estuaries (Nitinat Lake, Nanaimo River, and Campbell River, British Columbia; Columbia River, Oregon) were inhabited by a distinct, native North Pacific clade (Lee 2000). In 1913, Esterly (1924) reported E. carolleeae (as 'E. affinis') as abundant in brackish waters of San Francisco Bay. Possible vectors for its introduction include transplants of Eastern Oysters (Crassostrea virginica), stocking of fish such as Striped Bass (Morone saxatilis) or American Shad (Alosa sapidissima) in the 19th century, or a later introduction by ballast water. It is not clear whether E. carolleeae replaced a native population of the North Pacific clade of E. affinis, or filled an empty niche. In San Francisco Bay, E. affinis/carolleeae was a dominant species in low salinity waters of the Delta, extending into San Pablo Bay and the Central Bay in years of high river flow (Esterly 1924; Ambler et al. 1985; Orsi and Mecum 1986). However, the Asian copepods Sinocalanus doerri (1979) and later, Pseudodiaptomus forbesi (1987), partially replaced this species in brackish waters (Ambler et al. 1985; Meng and Orsi 1991; Bollens et al. 2011).
Invasion History on the East Coast:
Eurytemora carolleeae was probably introduced to the Great Lakes by ships using the St. Lawrence Seaway (Mills et al. 1993; Lee 2000). It was first collected in Lake Ontario in 1958, and reached Lake Superior by 1972. In the Lakes, it is most abundant in harbor areas (Mills et al. 1993).
Invasion History Elsewhere in the World:
In 2007, a genetic survey of E. affinis from the Gulf of Finland, Russia (Baltic Sea) found that some of the E. affinis from two bays in the inner Gulf (Luga and Neva Bays) matched the Atlantic subclade (now E. carolleeae), and were distinct from the native Baltic form (Alekseev et al. 2009). Some copepods from the Gulf of Riga, the Netherlands; Gironde, Loire and Seine estuaries in France; and the Dassau River estuary, Germany, show morphological characters of E. carolleeae, but have not yet been confirmed genetically (Sukhikh et al. 2013).
Description
Eurytemora carolleeae was recently separated from the circumboreal E. affinis species complex (Lee 2000; Alekseev & Souissi 2011; Sukhikh et al. 2019). The cryptic species and clades, found by Lee (1999; 2000) are difficult to distinguish morphologically (Lee 1999; Lee 2000; Lee and Frost 2002). However, close examination of a large number of features shows differences between E. carolleeae and European E. affinis (Alekseev & Souissi 2011).
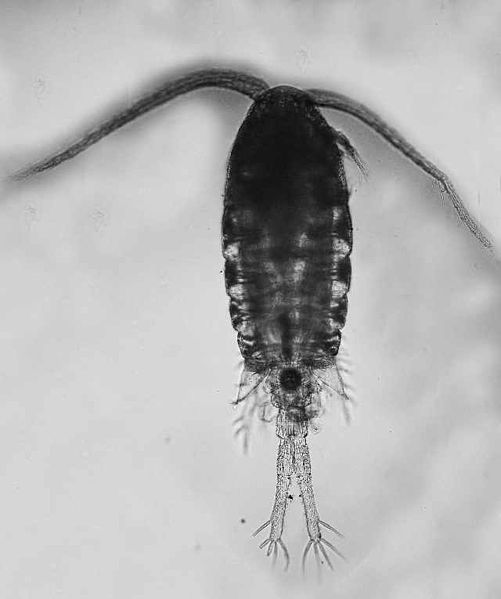
Adult females have six prosomal segments, of which the posterior most has large angular projections ('wings'), which reach beyond the genital segments. The caudal rami are elongated to about 7 X the width. The antennules (1st antennae) are short and curved convexly, and have 24 segments but are shorter than the prosome. The caudal rami and the last urosome segment are covered with dense spines (Alekseev and Souissi 2011). Two of the four urosome segments are fused to form the genital segment. In E. carolleeae, there are wing-like outgrowths on the fused genital segment (absent in European E. affinis from the type locality) (Alekseev and Souissi 2011; Sukhikh et al. 2013). The mid-lateral sides of the genital segment (Urosome segment 2) are expanded into short rounded processes. The coxae (basal segments) of swimming legs 1-4 have long setae at the inner side (Alekseev and Souissi 2011). The 3rd segments of the 5th swimming legs (P5, periopod, swimming leg) are stretched into broad, inward-pointing, thorn-like spurs, which are symmetrical and oriented under 45 degrees to the segment axes with two sub-equal spines (Alekseev and Souissi 2011). The eggs are carried in a single mass under the urosome. The overall length of the female is 1.14-1.48 mm (Gerber 2000; Johnson and Allen 2005).
Adult males also have six prosomal segments, but have a slender body, tapering rearwards, and lacking the 'wings' of the female form. Instead, the last prosomal segment is rounded. There are five fairly uniform urosomal segments. The caudal rami are long and narrow, about 10 X the width. The last urosomal segment and the sides of the rami are covered with fine setae, but the dorsal and ventral surfaces are naked (Alekseev and Souissi 2011). There are spines on the right antennule segments 10 and 11. The right antennule (1st antenna) is geniculate (jointed) at segment 19, and the 2 terminal segments (20, 21) beyond the joint, are 1.1 X the length of segment 19, proximal to the joint. The coxae of swimming legs P1-P4 have long setae on the inner sides of the coxae. The P5 legs are asymmetrical. The right leg is 4-segmented – the 2nd segment of right P5 has a triangular swelling (Gerber 2000; Johnson and Allen 2005). The basal segment of the left P5 is more or less cylindrical in shape, provided with two small rounded outgrowths (a larger triangular swelling occurs in E. affinis). The second (distal) segment of the left exopod has two lobes at the end (Alekseev and Souissi 2011).
Eurytemora affinis was described from the Elbe River in Germany, and reported from brackish and coastal fresh waters around the Northern Hemisphere. However, morphological and ecological differences have long been noted over the species' broad range, resulting in multiple named species of uncertain status (Lee 2000; Alekseev and Souissi 2011). Genetic analysis by Lee (2000) identified at least six distinct lineages, for Europe, Asia, North Pacific, Gulf of Mexico, and two sympatric (Atlantic and North Atlantic) from the East Coast of North America. The morphological differences among clades are small, compared to the genetic divergences among populations, complicating the taxonomy of this species complex (Lee and Frost 2002). A more extensive morphological analysis found several important morphological characters (noted above), which clearly separate the North American E. carolleeae from the European E. affinis sensu stricto (Alekseev and Souissi 2011).
Developmental stages of E. carolleeae (as E. affinis) from Woods Hole, Massachusetts are described and compared with E. americana and E. herdmanni by Katona (1971).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Arthropoda | |
| Subphylum: | Crustacea | |
| Class: | Maxillopoda | |
| Subclass: | Copepoda | |
| Order: | Calanoida | |
| Family: | Temoridae | |
| Genus: | Eurytemora | |
| Species: | carolleeae |
Synonyms
Eurytemora hirundo (Giesbrecht, 1881)
Eurytemora affinis (Poppe, 1880)
Eurytemora hirundoides (Nordquist, 1888)
Potentially Misidentified Species
This cryptic species was detected in the St. Lawrence River and Waquoit Bay, Massachusetts (MA). It overlaps with the native range of the Atlantic subclade, but is reproductively isolated from it. 'Atlantic' animals from Edgartown, MA crossed with 'North Atlantic' animals from Waquoit Bay, MA failed to produce viable offspring (Lee 2000). 'Atlantic' and 'North Atlantic' animals could not be distinguished morphologically (Lee and Frost 2002).
Eurytemora affinis (North Pacific subclade)
This cryptic species was detected in the Columbia River estuary and Nitinat Lake, British Columbia. It overlaps with an introduced population of the Atlantic subclade, but is reproductively isolated from it. 'Atlantic' animals from Grays Harbor, Washington (WA) crossed with 'North Pacific' animals from the Columbia River, failed to produce viable offspring (Lee 2000). 'Atlantic' and 'North Atlantic' animals could not be distinguished morphologically (Lee and Frost 2002).
Eurytemora affinis (senso stricto)
This cryptic species shows some morphological divergence from the North American clades of 'E. affinis' (Lee and Frost 2002). A detailed redescription of E. affinis from the type locality, the Elbe River, was made by Alekseev & Souissi (2011). It is not clear whether the other commonly used names (hirundo and hirundoides) refer to separate species. Sukhikh et al. (2016) have compared genetics and morphology E. affinis population from the Baltic to central France, and concluded that the the variation represented several subspecies.
Eurytemora americana
Native to NW Atlantic and NE Pacific; estuarine, but rare in completely fresh water; P5 structure in males and females is distinct from the E. affinis complex.
Eurytemora caspica
This cryptic species is native to the Caspian Sea (Sukhikh and Alekseev 2013)
Eurytemora herdmanni
Native to NW Atlantic, poly-euhaline, estuarine coastal
Eurytemora pacifica
Native to NE Pacific, poly-euhaline
Ecology
General:
Planktonic calanoid copepods mate in the water column. Males use their modified antenules and 5th pair of swimming legs to grasp the female and transfer spermatophores to the female's genital segment. Female Eurytemora spp. carry eggs in a single cluster under the abdomen (Katona 1971; Johnson and Allen 2005). Eggs hatch into nauplii which go through six stages. The first stage, NI, has 3 pairs of appendages and is unsegmented - each molt has additional appendages and/or more differentiation of segments. The sixth stage (NVI) molts into a first copepodite stage (CI), with the basic form of the adult and fully differentiated feeding structures, but with only two pairs of swimming legs and only one urosomal segment. The copepod goes through five additional molts, with increasing numbers of swimming legs, urosomal segments, and sexual differentiation. The sixth (CVI) stage is the male or female adult (Katona 1971; Barnes 1983). Time from hatching to maturity ranged from ~80 days at 5.5 C to 10 days at 25 C (Heinle and Flemer 1975).
Eurytemora carolleeae, like other copepods of the E. affinis complex, is characteristic of estuaries with low-salinity waters (Esterly 1924; Newell and Newell 1977; Lee 2000). It is capable of completing its life cycle in freshwater, and inhabits tidal fresh waters, freshwater coastal lakes, and has colonized the Laurentian Great Lakes (Lee 2000; Lee and Petersen 2002; Lee et al. 2004). Populations in the Great Lakes show rapid evolution of freshwater tolerance, coupled with a reduction in seawater tolerance (Lee et al. 2004). Egg-bearing females in calm waters tend to avoid surface waters by day and cluster around vegetation (Fofonoff, personal observations). All life stages feed on phytoplankton, although adults may also capture ciliates, rotifers, and copepod nauplii (Barnes 1983).
Food:
Phytoplankton; Protozoans; Detritus
Consumers:
Fishes; Medusae; Mysids;
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Fresh (nontidal) Marsh | None |
| General Habitat | Coarse Woody Debris | None |
| General Habitat | Nontidal Freshwater | None |
| General Habitat | Tidal Fresh Marsh | None |
| General Habitat | Unstructured Bottom | None |
| General Habitat | Marinas & Docks | None |
| Salinity Range | Limnetic | 0-0.5 PSU |
| Salinity Range | Oligohaline | 0.5-5 PSU |
| Salinity Range | Mesohaline | 5-18 PSU |
| Salinity Range | Polyhaline | 18-30 PSU |
| Salinity Range | Euhaline | 30-40 PSU |
| Tidal Range | Subtidal | None |
| Vertical Habitat | Epibenthic | None |
| Vertical Habitat | Planktonic | None |
Tolerances and Life History Parameters
| Minimum Temperature (ºC) | 0 | None |
| Minimum Salinity (‰) | 0 | Field and lab (Lee 2000) |
| Maximum Salinity (‰) | 40 | Field (Lee and Petersen 2002) |
| Minimum Reproductive Temperature | 5.5 | Lowest tested, Chesapeake Bay (Heinle and Flemer 1975) |
| Maximum Reproductive Temperature | 25 | Highest tested, Chesapeake Bay (Heinle and Flemer 1975) |
| Minimum Reproductive Salinity | 0 | Lake Michigan populations (Lee et al. 2004) |
| Maximum Reproductive Salinity | 27 | Highest tested, population from brackish Edgartown Great Pond MA (Lee and Petersen 2002); Lake Michigan populations had only ~20% survival to adulthood at 25 PSU (Lee et al. 2004). |
| Minimum Length (mm) | 1.1 | Adult total length (Gerber 2000; Johnson and Allen 2005) |
| Maximum Length (mm) | 1.5 | Adult total length (Gerber 2000; Johnson and Allen 2005) |
| Broad Temperature Range | None | Cold temperate-Warm temperate |
| Broad Salinity Range | None | Nontidal Limnetic-Euhaline |
General Impacts
The full impacts of the invasion of Eurytemora carolleeae in San Francisco Bay on the native zooplankton community are unknown. It is possible that the E. carolleeae filled an empty niche, with few brackish-water copepods being present, or it could have replaced a native population of the Pacific form of E. 'affinis' which was a numerically dominant copepod in the tidal-fresh to mesohaline regions of the San Francisco Bay estuary (Esterly 1927; Ambler 1986). The copepod 'E. affinis' was found in the earliest surveys of the estuary (Esterly 1927), but we have limited knowledge of the interactions between E. carolleeae and E. affinis within the bay. Research shows that E. 'affinis' was partially replaced by introduced Asian copepods, beginning in the 1970s, with the invasion of Sinocalanus doerri, and to a greater degree the invasion of Pseudodaiptomus forbesi (Baxter et al. 2007). The invasion of the clam Corbula amurensis also resulted in a dramatic decline in zooplankton abundance throughout the bay (Kimmerer et al. 1994).Regional Impacts
| P090 | San Francisco Bay | Ecological Impact | Food/Prey | ||
| Eurytemora carolleeae was a numerically dominant copepod in the tidal-fresh to mesohaline regions of the San Francisco Bay estuary (Esterly 1924; Ambler et al. 1986). It provided a major food source for mysids (Neomysis mercedis), in turn providing a major food source for juvenile fishes. but was largely replaced by introduced Asian copepods (Sinocalanus doerri, Pseudodiaptomus forbesi), beginning in the 1970s, with the invasion of S. doerri (Bryant and Arnold 2007). The invasion of the clam Corbula amurensis resulted in a dramatic decline in zooplankton abundance, due in part to the clam filtering out nauplii (Kimmerer et al. 1994). In feeding experiments with larval Striped Bass (Morone saxatilis), E. affinis was eaten at higher rates than the introduced P. forbesi or S. doerri (Meng and Orsi 1991). Eurytemora carolleeae was also a dominant food organism of the endangered Delta Smelt (Hypomesus transpacificus and the threatened Longfin Smelt (Spirinchus thaleichthys in the Sacramento-San Joaquin Delta, and considered a superior food to P. forbesi (Moyle et al. 1992; Nobriga 2002; Hamilton et al. 2020). The effects of introduced copepods are additionally complex, because of the varying size of the life-stages, and the interaction of different species of fish predators (Slater et al. 2014; Sullivan et al. 2016). | |||||
| NEP-V | Northern California to Mid Channel Islands | Ecological Impact | Food/Prey | ||
| Eurytemora carolleeae was a numerically dominant copepod in the tidal-fresh to mesohaline regions of the San Francisco Bay estuary (Esterly 1924; Ambler et al. 1986). It provided a major food source for mysids (Neomysis mercedis), in turn providing a major food source for juvenile fishes. but was largely replaced by introduced Asian copepods (Sinocalanus doerri, Pseudodiaptomus forbesi), beginning in the 1970s, with the invasion of S. doerri (Bryant and Arnold 2007). The invasion of the clam Corbula amurensis resulted in a dramatic decline in zooplankton abundance, due in part to the clam filtering out nauplii (Kimmerer et al. 1994). In feeding experiments with larval Striped Bass (Morone saxatilis), E. carolleeae was eaten at rates than the introduced P. forbesi or S. doerri (Meng and Orsi 1991). Eurytemora affinis was also a dominant food organism of the endangered Delta Smelt (Hypomesus transpacificus and the threatened Longfin Smelt (Spirinchus thaleichthys in the Sacramento-San Joaquin Delta, and considered a superior food to P. forbesi (Moyle et al. 1992; Nobriga 2002). The effects of introduced copepods are additionally complex, because of the varying size of the life-stages, and the interaction of different species of fish predators (Sullivan et al. 2016). | |||||
| P090 | San Francisco Bay | Ecological Impact | Herbivory | ||
| Eurytemora carolleeae was a numerically dominant copepod in the tidal-fresh to mesohaline regions of the San Francisco Bay estuary (Esterly 1924; Ambler et al. 1986). It was a major grazer on phytoplankton populations in the estuary, until its decline due to competition with introduced copepod species Sinocaanus doerri, Pseudodiaptomus forbesi), and the invasion of the suspension-feeding clam Corbula amurensis (Kimmerer et al. 1994). | |||||
| NEP-V | Northern California to Mid Channel Islands | Ecological Impact | Herbivory | ||
| Eurytemora carolleeae was a numerically dominant copepod in the tidal-fresh to mesohaline regions of the San Francisco Bay estuary (Esterly 1924; Ambler et al. 1986). It was a major grazer on phytoplankton populations in the estuary, until its decline due to competition with introduced copepod species Sinocaanus doerri, Pseudodiaptomus forbesi), and the invasion of the suspension-feeding clam Corbula amurensis (Kimmerer et al. 1994; Hamilton et al. 2020). | |||||
| B-IX | None | Ecological Impact | Competition | ||
| In some years (2010, 2015), Eurytemora carolleeae was the dominant, replacing native E. affinis in Neva Bay, Gulf of Finland, Russia, but was less abundant in the nearby Luga Bay, with lower summer temperatures, and higher fish predation. Eurytemora carolleeae has higher fecundity and larger egg-sacs, giving it a competitive advantage, but increasing vunlerabilty to fish predation (Sukhikh et al. 2019). | |||||
| CA | California | Ecological Impact | Food/Prey | ||
| Eurytemora carolleeae was a numerically dominant copepod in the tidal-fresh to mesohaline regions of the San Francisco Bay estuary (Esterly 1924; Ambler et al. 1986). It provided a major food source for mysids (Neomysis mercedis), in turn providing a major food source for juvenile fishes. but was largely replaced by introduced Asian copepods (Sinocalanus doerri, Pseudodiaptomus forbesi), beginning in the 1970s, with the invasion of S. doerri (Bryant and Arnold 2007). The invasion of the clam Corbula amurensis resulted in a dramatic decline in zooplankton abundance, due in part to the clam filtering out nauplii (Kimmerer et al. 1994). In feeding experiments with larval Striped Bass (Morone saxatilis), E. carolleeae was eaten at rates than the introduced P. forbesi or S. doerri (Meng and Orsi 1991). Eurytemora affinis was also a dominant food organism of the endangered Delta Smelt (Hypomesus transpacificus and the threatened Longfin Smelt (Spirinchus thaleichthys in the Sacramento-San Joaquin Delta, and considered a superior food to P. forbesi (Moyle et al. 1992; Nobriga 2002). The effects of introduced copepods are additionally complex, because of the varying size of the life-stages, and the interaction of different species of fish predators (Sullivan et al. 2016)., Eurytemora carolleeae was a numerically dominant copepod in the tidal-fresh to mesohaline regions of the San Francisco Bay estuary (Esterly 1924; Ambler et al. 1986). It provided a major food source for mysids (Neomysis mercedis), in turn providing a major food source for juvenile fishes. but was largely replaced by introduced Asian copepods (Sinocalanus doerri, Pseudodiaptomus forbesi), beginning in the 1970s, with the invasion of S. doerri (Bryant and Arnold 2007). The invasion of the clam Corbula amurensis resulted in a dramatic decline in zooplankton abundance, due in part to the clam filtering out nauplii (Kimmerer et al. 1994). In feeding experiments with larval Striped Bass (Morone saxatilis), E. affinis was eaten at higher rates than the introduced P. forbesi or S. doerri (Meng and Orsi 1991). Eurytemora carolleeae was also a dominant food organism of the endangered Delta Smelt (Hypomesus transpacificus and the threatened Longfin Smelt (Spirinchus thaleichthys in the Sacramento-San Joaquin Delta, and considered a superior food to P. forbesi (Moyle et al. 1992; Nobriga 2002; Hamilton et al. 2020). The effects of introduced copepods are additionally complex, because of the varying size of the life-stages, and the interaction of different species of fish predators (Slater et al. 2014; Sullivan et al. 2016). | |||||
| CA | California | Ecological Impact | Herbivory | ||
| Eurytemora carolleeae was a numerically dominant copepod in the tidal-fresh to mesohaline regions of the San Francisco Bay estuary (Esterly 1924; Ambler et al. 1986). It was a major grazer on phytoplankton populations in the estuary, until its decline due to competition with introduced copepod species Sinocaanus doerri, Pseudodiaptomus forbesi), and the invasion of the suspension-feeding clam Corbula amurensis (Kimmerer et al. 1994; Hamilton et al. 2020)., Eurytemora carolleeae was a numerically dominant copepod in the tidal-fresh to mesohaline regions of the San Francisco Bay estuary (Esterly 1924; Ambler et al. 1986). It was a major grazer on phytoplankton populations in the estuary, until its decline due to competition with introduced copepod species Sinocaanus doerri, Pseudodiaptomus forbesi), and the invasion of the suspension-feeding clam Corbula amurensis (Kimmerer et al. 1994). | |||||
Regional Distribution Map
Non-native
Native
Cryptogenic
Failed
| Bioregion | Region Name | Year | Invasion Status | Population Status |
|---|---|---|---|---|
| NA-S3 | None | 1994 | Native | Established |
| NA-ET2 | Bay of Fundy to Cape Cod | 0 | Native | Established |
| NA-ET3 | Cape Cod to Cape Hatteras | 0 | Native | Established |
| CAR-VII | Cape Hatteras to Mid-East Florida | 0 | Native | Established |
| NEP-V | Northern California to Mid Channel Islands | 1913 | Non-native | Established |
| NEP-IV | Puget Sound to Northern California | 1998 | Non-native | Established |
| GL-III | Lake Ontario | 1958 | Non-native | Established |
| GL-II | Lake Erie | 1961 | Non-native | Established |
| GL-I | Lakes Huron, Superior and Michigan | 1966 | Non-native | Established |
| P090 | San Francisco Bay | 1913 | Non-native | Established |
| P280 | Grays Harbor | 1998 | Non-native | Established |
| P093 | _CDA_P093 (San Pablo Bay) | 1913 | Non-native | Established |
| B-IX | None | 2007 | Non-native | Established |
| B-VIII | None | 2008 | Non-native | Unknown |
| NEA-III | None | 1931 | Non-native | Established |
| NEA-II | None | 2009 | Non-native | Established |
| B-IV | None | 2013 | Non-native | Established |
| B-V | None | 2013 | Non-native | Established |
| B-VII | None | 2013 | Non-native | Established |
| NEP-III | Alaskan panhandle to N. of Puget Sound | 1998 | Non-native | Established |
Occurrence Map

| OCC_ID | Author | Year | Date | Locality | Status | Latitude | Longitude |
|---|---|---|---|---|---|---|---|
| 34160 | Esterly 1924 | 1913 | 2005-01-01 | Delta General Location | Non-native | 38.0500 | -121.8100 |
References
Alekseev, V. R.; Abramson, N. I.; Sukhikh, N. M. (2009) Introduction of sibling species to the ecosystem of the Baltic Sea, Doklady Biological Sciences 429: 544-547Alekseev, Victor R.; Souissi, Anissa (2011) A new species within the Eurytemora affinis complex (Copepoda: Calanoida) from the Atlantic Coast of USA, with observations on eight morphologically different European populations, Zootaxa 2767: 41-56
Ambler, Julie W.; Cloern, James E.; Hutchinson, Anne (1985) Seasonal cycles of zooplankton from San Francisco Bay., Hydrobiologia 129: 177-197
Baxter, Randall, and 11 Authors (2008) <missing title>, Inter-Agency Ecological Program, Sacramento. Pp. 1-52
Bollens, Stephen M.; Breckenridge, Joanne K.; Cordell, Jeffery R. Simenstad, Charles A.; Kalata, Olga (2014) Zooplankton of tidal marsh channels in relation to environmental variables in the upper San Francisco Estuary, Aquatic Biology 21: 205-219
Bollens, Stephen M.; Breckenridge, Joanne K. Vanden Hoof, Rian C.; Cordell, Jeffery R. (2011) Mesozooplankton of the lower San Francisco Estuary: spatio-temporal patterns, ENSO effects and the prevalence of non-indigenous species, Journal of Plankton Research 33(9): 1358-1377
Bowman, Thomas E., Lewis, Julian J. (1989) Occurrence of the calanoid copepod Eurytemora affinis (Poppe) in the Ohio River at Louisville, Kentucky, Journal of Crustacean Biology 9(1): 83-84
Ching, Chau Fong (2007) <missing title>, University of Wisconsin (Master's Thesis), Madison. Pp. <missing location>
Cordell, Jeffrey (2012) A handbook of global freshwater invasive species, Eatrhscan, New York NY. Pp. 161-172
Counahan, Daniel F.; Carline, Robert F.; Reid, Janet W. (2005) The occurrence of the calanoid copepod Eurytemora affinis in the upper Ohio River, Northeastern Naturalist 12(4): 541-545
Crivellaro, Marcelo Schuler and 6 auhtors (2021) Fighting on the edge: reproductive effort and population structure of the invasive coral Tubastraea coccinea in its southern Atlantic limit of distribution following control activities, Biological Invasions <missing volume>: <missing location>
Croskery, Peter (1978) The freshwater co-occurrence of Eurytemora affinis (Copepoda: Calanoida) and Manayunkia speciosa (Annelida: polychaeta): possible reficts of a marine incursion., Hydrobiologia 59(3): 237-241
Dodson, Stanley I.; Skelly, Daniel A.; Lee, Carol Eunmi (2010) Out of Alaska: morphological diversity within the genus Eurytemora from its ancestral Alaskan range (Crustacea, Copepoda), Hydrobiologia 653: 131-148
Esterly, Calvin O. (1924) The free-swimming Copepoda of San Francisco Bay, University of California Publications in Zoology, Berkeley 26(5): 81-129
Fisher, Jonathan A. D.; Rhile, Erika C.; Harrison, Liud; Petraitis, Peter S. (2009) An intertidal snail shows a dramatic size increase over the past century, Proceedings of the National Academy of Sciences 106(13): 5209-5212
Gauff, Robin (2023) Unexpected biotic homogenization masks the effect of a pollution gradient on local variability of community structure in a marine urban environment, Journa of Experimental Marine Biology and Ecology 562(151882): Published online
https://doi.org/10.1016/j.jembe.2023.151882
Ger, Kemal Ali; Arneson, Patricia; Goldman, Charles Remington; Joo Teh, Swee (2010) Species specific differences in the ingestion of Microcystis cells by the calanoid copepods Eurytemora affinis and Pseudodiaptomus forbesi, Journal of Plankton Research 32(10): 1479-1484
Gerber, Ray P. (2000) <missing title>, 1 & 2 Acadia Productions, Brunswick ME. Pp. <missing location>
Gittenberger, Arjan; Mirimin, Luca; Boyd, John; O’Beirn, Francis; Devine, Grainne; O’Brien, Martina; Rensing, Marjolein; O’Dwyer, Katie; Gittenberger, Edmund (2023) Marine Non-Indigenous Species Dynamics in Time and Space within the Coastal Waters of the Republic of Ireland, Diversity 15(1019): Published online
https:// doi.org/10.3390/d15091019
Hamilton, Scott; Bartell, Steve; Pierson, James; Murphy, Dennis (2021) Factors controlling calanoid copepod biomass and distribution in the Upper San Francisco Estuary and Implications for Managing the imperiled Delta Smelt (Hypomesus transpacificus), Environmental Management 65: 587-601
Havel, John E.; Lee, Carol Eunmi; Vander Zanden, Jake (2005) Do reservoirs facilitate invasions into landscapes?, BioScience 55(6): 518-525
Heinle, D. R.; Flemer, D. A. (1975) Carbon requirements of a population of the estuarine copepod Eurytemora affinis, Marine Biology 31: 235-247
Hutchings, Brenna; Emma Stiles; Erwin, Patrick M () Hurricane events facilitate the dominance of introduced invertebrate species in harbors, Biological Invasions <missing volume>: Published online
https://doi.org/10.1007/s10530-023-03056-w
Johnson, William S.; Allen, Dennis M. (2005) <missing title>, Johns Hopkins Press, Baltimore. Pp. <missing location>
Katona, S. K. (1971) The developmental stages of Eurytemora affinis (Poppe, 1880) (Copepoda, Calanoida) raised in laboratory cultures, including a comparison with the larvae of Eurytemora americana Williams, 1906, and Eurytemora herdmani Thompson & Scott, Crustaceana 21(1): 5-20
Kimmerer, William J.; Burau, J. R.; Bennett, W. A. (1998) Tidally oriented vertical migration and position maintenance of zooplankton in a temperate estuary., Limnology and Oceanography 43(7): 1697-1709
Kimmerer, William; Gartside, Ellen; Orsi, James J. (1994) Predation by an introduced clam as the likely cause of substantial declines in zooplankton of San Francisco Bay., Marine Ecology Progress Series 113: 81-93
Kimmerer, Wim J.; Lougee, Laurence (2015) Bivalve grazing causes substantial mortality to an estuarine copepod population, Journal of Experimental Marine Biology and Ecology 473: 53-63
Lee, Carol E. (2000) Global phylogeography of a cryptic copepod species complex and reproductive isolation between genetically proximate populations., Evolution 54(6): 2014-2027
Lee, Carol Eunmi (1999) Rapid and repeated invasions of fresh water by the copepod Eurytemora affinis., Evolution 53(5): 1423-1434
Lee, Carol Eunmi and 5 authors (2013) Feasting in fresh water: impacts of food concentration on freshwater tolerance and the evolution of food X salinity response during the expansion from saline into fresh water habitats, Evolutionary Applications 6: 673-684
Lee, Carol Eunmi; Bell, Michael A. (1999) Causes and consequences of recent freshwater invasions by saltwater animals, Trends in Ecology and Evolution 14(7): 284-288
Lee, Carol Eunmi; Frost, Bruce W. (2002) Morphological stasis in the Eurytemora affinis species complex (Copepoda: Temoridae)., Hydrobiologia 480: 111-128
Lee, Carol Eunmi; Kiergaard, Michael; Gelembiuk, Gregory William;Eads, Brian Donovan; Posavi, Marijan (2011) Pumping ions: Rapid parallel evolution of ionic regulation following habitat invasions, Evolution 65(8): 2229-2244
Lee, Carol Eunmi; Petersen, Christine H. (2002) Genotype-by-environment interaction for salinity tolerance in the freshwater-invading copepod Eurytemora affinis, Physiological and Biochemical Zoology 75(4): 335-344
Meng, Lesa; Orsi, James J. (1991) Selective predation by larval striped bass on native and introduced copepods., Transactions of the American Fisheries Society 120: 187-192
Mills, Edward L.; Leach, Joseph H.; Carlton, James T.; Secor, Carol L. (1993) Exotic species in the Great Lakes: a history of biotic crises and anthropogenic introductions., Journal of Great Lakes Research 19(1): 1-54
Mizrahi, Damián; Silva, Milena C.; Fonseca, Maurício L.; Lopes, Rubens M. (2023) Resistance to desiccation and healing regeneration in the sun coral, Bioinvasions Records 14: In press
Montie, Shinae; Thomsen, Mads S. (2023) Long-term community shifts driven by local extinction of an iconic foundation species following an extreme marine heatwave, Ecology and Evolution 13(e10235): Published online
https://doi.org/10.1002/ece3.10235
Moyle, Peter B.; Herbold, Bruce; Stevens, Donald E.; Miller, Lee W. (1992) Life history and status of the delta smelt in the Sacramento-San Joaquin estuary, California, Transactions of the American Fisheries Society 121: 67-77
Nobriga, Matthew L. (2002) Larval delta smelt diet composition and feeding incidence: Environmental and ontogenetic influences, California Fish and Game 88(4): 149-164
Orsi, James J.; Mecum, Walter L. (1986) Zooplankton distribution and abundance in the Sacramento-San Joaquin Delta in relation to certain environmental factors, Estuaries 9(4B): 326-339
Orsi, Jim (2001) Eurytemora affinis is introduced., IEP Newsletter 14(4): 12
Otani, Michio; Yamanishi, Ryohei (2010) Distribution of the alien species Hydroides dianthus (Verrill, 1873) (Polychaeta: Serpulidae) in Osaka Bay, Japan, with comments on the factors limiting its invasi, Planton and Benthios Research 5(2): 62-68
Raffo, M. Paula; Eyras, M. Cecilia; Iribarne, Oscar O. I (2009) The invasion of Undaria pinnatifida to a Macrocystis pyrifera kelp in Patagonia (Argentina, south-west Atlantic), Journal of the Marine Biological Association of the United Kingdom 89(8): 1571-1580.
Reid, Janet L.; Hudson, Patrick (2008) Comment on 'Rate of species introductions in the Great Lakes via ships' ballast water and sediments', Canadian Journal of Fisheries and Aquatic Science 65: 549-553
Ruiz, G.; Chang, A.; McCann, L.; Larson, K.; DeJesus, J.; Lion, K.; Ashton, G.; Blumenthal, J.; Hitchcock, N.; Havard, S.; Keppel, E.; Steves, B.; Muirhead, J.; Pappalardo, P.; Fofonoff, P.; Fofonoff P.; Geller, J.; DiMaria, R.; Arena, M.; Lohan, K Pagenkopp (2024) Regional Evaluation of Non-indigenous Marine Species in Prince William Sound: Final Report to Prince William Sound Regional Citizens’ Advisory Council (PWSRCAC). https://www.pwsrcac.org/document/regional-evaluation-of-non-indigenous-marine-species-in-prince-william-sound/
Ruiz, Gregory; Geller, Jonathan (2021) Spatial and temporal analysis of marine invasions: supplemental studies to evaluate detection through quantitative and molecular methodologies, Marine Invasive Species Program, California Department of Fish and Wildlife, Sacramento CA. Pp. 153 ppl.
Saunders, James F., Jr. (1993) Distribution of Eurytemora affinis (Copepoda: Calanoida) in the southern Great Plains, with notes on zoogeography, Journal of Crustacean Biology 13(3): 564-570
Slugocki, Lukasz; Rymaszewska, Anna; Kirczuk, Lucyna , (2021) To fit or to belong: characterization of the non-native invader Eurytemora carolleeae (Copepoda: Calanoida) in the Oder River system (Central Europe), Bioinvasions Records 16: 443–460)
Strasser, Carly A.; Lewis, Mark A.; DiBacco, Claudio (2011) A mechanistic model for understanding invasions: using the environment as a predictor of population success, Diversity and Distributions published online: 1-15
Sukhikh, N. M.; Alekseev, V. R. (2013) Eurytemora caspica sp. nov. from the Caspian Sea: One more new species within the E. affinis complex (Copepoda: Calanoida: Temoridae), Proceedings of the Zoological Institute RAS 429: 544-547
Sukhikh, Natalia; Souissi, Anissa; Souissi, Sami; Alekseev, Victor (2013) Invasion of Eurytemora sibling species (Copepoda: Temoridae) from North America into the Baltic Sea and European Atlantic coast estuaries, Journal of Natural History 47(5-12): 753-767
Trott, Thomas J.; Enterline, Claire (2019) First record of the encrusting bryozoan Cribrilina ( Juxtacribrilina ) mutabilis (Ito, Onishi and Dick, 2015) in the Northwest Atlantic Ocean, BioInvasions Records 8: 598-607
Vasquez, Adrian A.; Hudson, Patrick L.; Fujimoto, Masanori Keeler, Kevin Armenio, Patricia M. Rama, Jeffrey L. (2016) Eurytemora carolleeae in the Laurentian Great Lakes revealed by phylogenetic and morphological analysis, Journal of Great Lakes Research 42: 802-811
Ware, Chris et al. (2015) Biological introduction risks from shipping in a warming Arctic, Journal of Applied Ecology Published online: <missing location>
Wilson, Mildred Stratton, Yeatman, Harry C. (1959) <missing title>, <missing publisher>, <missing place>. Pp. 735-794
Winder; Monika; Jassby, Alan D.; Mac Nally, Ralph (2011) Synergies between climate anomalies and hydrological modifications facilitate estuarine biotic invasions, Ecology Letters 14: 749-757
Winkler, Gesche; Dodson, Julian J.; Lee, Carol Eunmi (2008) Heterogeneity within the native range: population genetic analyses of sympatric invasive and noninvasive clades of the freshwater invading copepod Eurytemora affinis., Molecular Ecology 17: 415-430
Winkler, Gesche; Souissi, Sami; Poux, Celine; Castric, Vincent (2011) Genetic heterogeneity among Eurytemora affinis populations in Western Europe, Marine Biology 158: 1841-1856