Invasion History
First Non-native North American Tidal Record: 1881First Non-native West Coast Tidal Record:
First Non-native East/Gulf Coast Tidal Record: 1881
General Invasion History:
Sphaeroma terebrans was described by Bate in 1866, from Brazil (Richardson 1905). It is now widely distributed in tropical-subtropical regions, including the Indo-Pacific from Taiwan to Australia; South Africa and along the West coast of Africa; and the Atlantic coast of South and North America, where it has been collected as far north as Virginia (Schultz 1969; Harrison and Holdich 1989; Kensley and Schotte 1989; Davidson et al. 2014). It was originally described from Brazil in 1866, but its close affinity to Indian Ocean species, its absence of planktonic larvae, and the well documented recent introductions of related Indo-Pacific species (S. annandalei, S. walkeri, S. quoianum) indicate that S. terebrans was probably transported to the Atlantic on the hulls of wooden ships before 1850 (Carlton and Ruckelshaus 1997). Carlton and Ruckelshaus (1997) predict that no evidence of S. terebrans boring into mangrove roots will be found in sediments predating European colonization. Genetic analysis indicates that 'S. terebrans' is a complex of at least four species (Baratti et al. 2005; Baratti et al. 2011). Two of these clades (A and B) coexist on the Indian Ocean coast of Africa. Populations in Florida and Brazil were assigned to Clade C, closely related to Clade B. Baratti et al. (2005 and 2011) attribute the global distribution of these forms, with subsequent speciation to natural dispersal, by rafting. However, they have examined only a small portion of the range of S. terebrans and cannot exclude the possibility that species C exists elsewhere in the Indo-Pacific.
North American Invasion History:
Invasion History on the East Coast:
The first North American record of Sphaeroma terebrans (as S. destructor), that we know of, was from Crescent City, Florida, near the headwaters of the St. Johns River in 1881 (US National Museum of Natural History 2009). Another collection was made in the Colleton River, South Carolina in 1891 (US National Museum of Natural History 2009). This isopod was formally described as S. destructor from the pilings of a bridge in freshwaters of the St. Johns River, Palatka, Florida (Richardson 1897). It was collected near Lake Pontchartrain, Louisiana, in 1906 (US National Museum of Natural History 2009). The regular range of this isopod appears to be from South Carolina to Texas, and southward to Cuba, Belize, and Brazil (Richardson 1897; Richardson 1905; Wallour 1960; Menzies and Frankenberg 1966; Miller 1968; Kensley and Schotte 1989). In Florida, S. terebrans is distributed around much of the peninsula, but was absent from about half of the 51 sites surveyed, including the Keys and the southernmost tip (Conover and Reid 1975). Sphaeroma terebrans was recorded only once from Chesapeake Bay, from the hull of a boat in Urbanna, Virginia, near the mouth of the Rappahannock River (Miller 1968; Van Engel 1972; United States National Museum of Natural History collections). It was considered a 'stray, unlikely to become established' (Van Engel 1972).
Invasion History Elsewhere in the World:
Outside the continental United States, Sphaeroma terebrans is found in Mexico (Montalvo-Urgel et al. 2010); Cuba (1994, USNM 280038, US National Museum of Natural History 2009); Belize (1980, USNM 205619, US National Museum of Natural History; Davidson et al. 2016); Costa Rica (Villalobos et al. 1985); Colon, Panama (Caribbean, Davidson et al. 2016); and Venezuela (1987, USNM 234047, US National Museum of Natural History 2009). We follow Carlton and Ruckelshaus (1997) in considering S. terebrans a likely very early introduction to the tropical Western Atlantic. However, paleontological studies of mangrove communities, and genetic studies are desirable to clarify the history and origin of this isopod.
Description
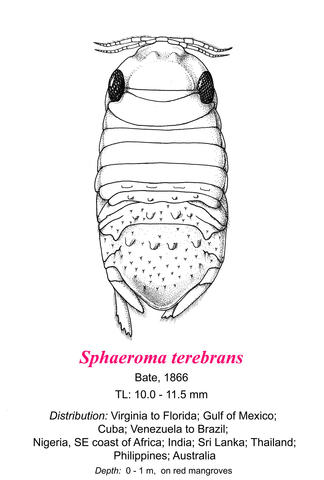
Sphaeroma terebrans has a compact, convex, elliptical body, which is about twice as long as it is wide. The posterior-dorsal surface of the body, and particularly the pleoteloson, is covered with tubercles. The head is approximately semicircular, with prominent eyes, composed of many ocelli. Antenna 1 has a flagellum of 11 segments and extends to the posterior edge of Peraeonite 1. Antenna 2's flagellum has 16 segments and reaches the posterior edge of Peraeonite 2. The peraeonites are roughly equal in width. Peraeonites 2-4 each bear a broad transverse ridge, while Peraeonites 4, 6, and 7 each bear four large tubercles. The pleotelson is roughly triangular and covered with tubercles. Many of the tubercles bear clusters of hairs. Pereiopods 1-3 bear dense plumose setae on the upper (anterior) surface of segments 3-4, an adaptation for filter-feeding. The outer edges of the exopods of the uropods each bear five prominent teeth. Adults are 8-12 mm long and are brown-to-reddish brown. They frequently roll into balls when disturbed. This description is based on: Richardson 1905, Van Name 1936, Schultz 1969, Harrison and Holdich 1984, Kensley and Schotte 1989, Theil 1999, and Hossain and Bamber 2013.
Isopods identified as S. terebrans are widely distributed in subtropical and tropical waters, and show substantial morphological and genetic variation. Specimens from the Western Atlantic were described as S. destructor (Richardson 1897, cited by Richardson 1905). Sphaeroma terebrans is usually treated as synonymous with S. destructor (Van Name 1936; Miller 1968; Harrison and Holdich 1984), but sometimes with uncertainty (Kensley and Schotte 1989). Genetic studies (Baratti et al. 2005; Baratti et al. 2011) indicate a high degree of genetic diversity, suggesting that 'S. terebrans' is a complex of several species. However, some of the sharpest differences are between Clades A and B, both on the coast of East Africa, while the Western Atlantic clade (Florida-Brazil) is closely related to Clade B. The number of sites sampled was relatively small, compared to the large range of the species. Therefore, further genetic studies are needed to describe the genetic diversity and biogeographic history of the species.
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Arthropoda | |
| Subphylum: | Crustacea | |
| Class: | Malacostraca | |
| Subclass: | Eumalacostraca | |
| Superorder: | Peracarida | |
| Order: | Isopoda | |
| Suborder: | Flabellifera | |
| Family: | Sphaeromatidae | |
| Genus: | Sphaeroma | |
| Species: | terebrans |
Synonyms
Sphaeroma tenebrans (Richardson, 1905)
Sphaeroma destructor (Richardson, 1897)
Sphaeroma tuberculatoicrinitum (Hilgendorf, 1879)
Sphaeroma vastator (Bate, 1866)
Potentially Misidentified Species
Sphaeroma quadridentatum is native to the Northwest Atlantic, and is abundant in algal and fouling communities (Schultz 1969).
Sphaeroma walkeri
Sphaeroma walkeri is native to the Indo-Pacific, but widely introduced in subtropical and tropical areas around the world (Kensley and Schotte 1989).
Ecology
General:
Sphaeroma terebrans has separate sexes, with internal fertilization, brooded young and direct development. Females carry 10-80 embryos, with broods tending to increase with body size from 7.5 to 12 mm body length. Sphaeroma terebrans exhibits extended parental care, in which early juveniles remain in the parental burrow until they develop the ability to burrow and filter-feed. Females usually host 5-20 juveniles in their burrows (extremes 1-58). Juveniles in burrows were usually 2-3 mm in size. Males do not remain in the females' burrows after copulation. In the Indian River Lagoon, Florida, juveniles were scarce in January through March, and most abundant and June (Thiel 1999).
Sphaeroma terebrans occurs in warm-temperate to tropical climates, and tidal fresh to euhaline waters (Richardson 1905; Van Name 1936; Kensley and Schotte 1989; Wilkinson 2002). It bores into mangrove roots, Bald Cypress roots, and roots of fresh-brackish marsh plants (at least six different species). Borers were found in intertidal and shallow subtidal pilings, rotten wood, and Styrofoam floats (Richardson 1905; Rehm and Humm 1973; Estevez 1994; Wilkinson 2002). In more saline waters in Florida and the Caribbean, the most common substrates are the prop roots of Red Mangrove (Rhizophora mangle) (Rehm and Humm 1973; Brooks 2004; Brooks and Bell 2005; Davidson et al. 2016). This isopod is also occasionally found in branches and roots of White (Laguncularia racemosa) and Black Mangroves (Avicennia nitida). Burrow length is usually correlated with isopod size and the largest burrows reported (9-12 mm) were made by isopods 7-9 mm long. Animals from brackish-water areas had normal burrows at low (3 PSU) and medium salinities, but greatly reduced burrowing at high (30 PSU) salinity. However, effects of long-term acclimation and genetic variation were not examined (John 1970). There has been some debate as to whether S. terebrans obtains any nutrition from the wood or plant material that it consumes. A recent morphological study concluded that S. terebrans primary feeds by suspension-feeding on detritus and phytoplankton, and uses the wood primarily as shelter (Si et al. 2002). This isopod's burrow may limit predation.
A commensal isopod, Iais floridana has been found living in the burrows of S. terebrans in the Indian River Lagoon, Florida. This isopod has not been found elsewhere, but it closely resembles the Indo-Pacific I. singaporensis (Kensley and Schotte 1999). We consider I. floridana a likely introduction. Its effects on S. terebrans have not been studied, but a similar commensal, I. californica had no effects on the growth and survival of its host, S. quoianum, introduced to the West Coast (Rotramel 1975). Another, more unusual interaction with an isopod species, occurs in Florida, when juveniles of S. quadridentata invade the burrows of female S. terebrans, apparently benefiting from the other species parental care. The presence of the invading juveniles may decrease the duration of parental care for the females' own juveniles (Thiel 2000).
Food:
Phytoplankton, Detritus, Wood?
Trophic Status:
Suspension Feeder
SusFedHabitats
| General Habitat | Nontidal Freshwater | None |
| General Habitat | Tidal Fresh Marsh | None |
| General Habitat | Marinas & Docks | None |
| General Habitat | Coarse Woody Debris | None |
| General Habitat | Mangroves | None |
| General Habitat | Vessel Hull | None |
| Salinity Range | Limnetic | 0-0.5 PSU |
| Salinity Range | Oligohaline | 0.5-5 PSU |
| Salinity Range | Mesohaline | 5-18 PSU |
| Salinity Range | Polyhaline | 18-30 PSU |
| Salinity Range | Euhaline | 30-40 PSU |
| Tidal Range | Subtidal | None |
| Tidal Range | Low Intertidal | None |
| Vertical Habitat | Epibenthic | None |
Tolerances and Life History Parameters
| Minimum Temperature (ºC) | 8 | Field, Bonnet-Carre Spillway, Lake Pontchartrain LA (Wilkinson 2002) |
| Maximum Temperature (ºC) | 34 | Fort Pierce, Florida (Field, Thiel 1999) |
| Minimum Salinity (‰) | 0 | Field (Richardson 1897). |
| Maximum Salinity (‰) | 39 | Field data, Florida Bay (Brooks 2004) |
| Minimum Length (mm) | 9.5 | Van Name 1936 |
| Maximum Length (mm) | 11.5 | Female (Van Name 1936; Kensley and Schotte 1989) |
| Broad Temperature Range | None | Warm temperate-tropical |
| Broad Salinity Range | None | Fresh-Euhaline |
General Impacts
In Florida and other tropical regions, Sphaeroma terebrans plays an important role by recycling dead wood (Becker 1971) and regulating the growth of mangroves (Rehm and Humm 1973; Ribi 1982; Simberloff et al. 1978; Perry and Brusca 1989; Davidson et al. 2014), and also as a borer in tidal marsh vegetation (Estevez 1994). This isopod is also an economically important borer on submerged wooden structures, such as pilings (Atwood 1920; Becker 1971; Wilkinson et al. 2002). It has been found burrowing in Styrofoam floats, and damaging them in Lake Pontchartrain, Louisiana; Colon, Panama; the Philippines and Taiwan (Wilkinson et al. 2002; Davidson 2012).Herbivory- S. terebrans does not consume wood directly, but uses it for shelter, tunneling into it and filter-feeding, and also probably feeding on bacteria, fungi etc. growing on the tunnel walls (Becker 1971; Estevez 1945). However, a cellulase has been reported from some wood-boring Sphaeroma spp, so direct consumption of wood cannot be ruled out (Becker 1971). A more recent morphological study concludes that S. terebrans primary mode of feeding is suspension-feeding on detritus and phytoplankton (Si et al. 2002). Sphaeroma terebrans burrows into the aerial roots of Rhizophora mangle (Red Mangrove) (Humm and Rehm 1973), hollowing them out, and also into the rhizomes of Juncus roemerianus (Black Needle Rush) plants in tidal marshes (Estevez 1994).
Habitat Change – S. terebrans clearly has an important role in the dynamics of Rhizophora mangle (Red Mangrove) communities, but the precise nature of its effects on mangrove communities has been subject of study and debate. Initially, it was regarded as a destroyer of mangroves (Humm and Rehm 1973), but S. terebrans were also found to increase the frequency of root branching (Simberloff et al. 1978) and to differentially infest roots farther from established roots, resulting in increased density of roots near the stand (Ribi 1982). However, Perry and Brusca (1989), working with the similar Pacific S. peruvianum, found that the mangrove's responses to herbivory did not offset the loss of productivity due to Sphaeroma's burrowing. Sphaeroma terebrans effects on marsh vegetation could also encourage marsh erosion (Estevez 1994).
Regional Impacts
| CAR-I | Northern Yucatan, Gulf of Mexico, Florida Straits, to Middle Eastern Florida | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red mangrove (Rhizophora mangle) was found with 70-100% of mangrove roots infested, and 20-100% of roots severed (Rehm and Humm 1973; Conover and Reid 1975). Extensive burrowing was also found in tidal brackish and fresh marshes of tributaries of Tampa Bay and Charlotte Harbor, in peat and roots of Juncus plants. Burrows were especially common on the scarps of receding marshes, and could be accelerating marsh retreat (Estevez 1994). In experiments, R. mangle responds to boring activity by increasing the number of root endings in unparasitized roots, mostly those already buried in soil. Sphaeroma terebrans' boring activity could actually increase the stability of shorelines (Simberloff et al. 1978; Ribi 1982). However, in caging experiments in Rookery Bay (Bear Creek and Environmental Learning Center) and Braden River, exclusion of S. terebrans resulted in much more extensive growth and complexity of mangrove roots. Boring by S. terebrans may limit the seaward growth of the mangrove community (Davidson et al. 2016). Boring by S. terebrans in plastic foam floats contributes to pollution by plastic particles, with adverse consequences to marine foodwebs (Davidson 2012). In Lake Pontchartrain, Louisiana, S. terebrans extensively bored into Bald Cypress (Taxodium distichum) stumps, honeycombing them, and contributing to their decay and shoreline erosion (Wilkinson 2002). | |||||
| CAR-I | Northern Yucatan, Gulf of Mexico, Florida Straits, to Middle Eastern Florida | Economic Impact | Shipping/Boating | ||
| Sphaeroma terebrans has caused damage to docks, pilings, and boat hulls (Atwood 1932). Damage to plastic floats was extensive in southwest Florida and in Lake Pontchartrain LA (Davidson 2012). | |||||
| G070 | Tampa Bay | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red mangrove (Rhizophora mangle) was found with 70-80% of mangrove roots infested, and 20-40% of roots severed (Conover and Reid 1975). In caging experiments in Rookery Bay (Bear Creek and Environmental Learning Center) and Braden River, exclusion of S. terebrans resulted in much more extensive growth and complexity of mangrove roots (Davidson et al. 2016). Extensive burrowing was also found in tidal brackish and fresh marshes, in peat and roots of Juncus plants. Burrows were especially common on the scarps of receding marshes, and could be accelerating marsh retreat (Estevez 1994). Boring by S. terebrans in plastic foam floats contribute to pollution by plastic particles, with adverse consequences to marine foodwebs (Davidson 2012). | |||||
| G074 | _CDA_G074 (Crystal-Pithlachascotee) | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 90% of mangrove roots infested, and 60% of roots severed (Conover and Reid 1975). | |||||
| G050 | Charlotte Harbor | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 90-100% of mangrove roots infested, and 50-80% of roots severed (Conover and Reid 1975). | |||||
| G045 | _CDA_G045 (Big Cypress Swamp) | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 80-100% of mangrove roots infested, and 20-80% of roots severed (Conover and Reid 1975). | |||||
| G030 | North Ten Thousand Islands | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 100% of mangrove roots infested, and 80-100% of roots severed (Rehm and Humm 1973; Conover and Reid 1975). In caging experiments in Rookery Bay (Environmental Learning Center, Bear Creek), exclusion of S. terebrans resulted in much more extensive growth and complexity of mangrove roots (Davidson et al. 2016). | |||||
| G020 | South Ten Thousand Islands | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 100% of mangrove roots infested, and 80-100% of roots severed (Conover and Reid 1975). | |||||
| G010 | Florida Bay | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 70% of mangrove roots infested, and 20% of roots severed (Conover and Reid 1975). | |||||
| S200 | Biscayne Bay | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 80-100% of mangrove roots infested, and 60-100% of roots severed (Conover and Reid 1975). However, in experiments, R. mangle responds to boring activity by increasing the number of root endings in unparasitized roots, mostly those already buried in soil. Sphaeroma's boring activity could actually increase the stability of shorelines Simberloff et al. 1978; Ribi 1982). | |||||
| S196 | _CDA_S196 (Cape Canaveral) | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 100% of mangrove roots infested, and 90% of roots severed (Conover and Reid 1975). | |||||
| S190 | Indian River | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 80-100% of mangrove roots infested, and 30-60% of roots severed (Conover and Reid 1975). | |||||
| CAR-III | None | Ecological Impact | Habitat Change | ||
| In field observations in Costa Rica, Red Mangrove (Rhizophora mangle responds to boring activity by increasing the number of root endings in unparasitized roots, mostly those already buried in soil. Sphaeroma's boring activity could actually increase the stability of shorelines (Simberloff et al. 1978; Ribi 1982; Villalobos et al. 1985). However, in caging experiments in Galeta and Boca del Drago, Panama, exclusion of S. terebrans resulted in much more extensive growth and complexity of mangrove roots (Davidson et al. 2016). | |||||
| G070 | Tampa Bay | Economic Impact | Shipping/Boating | ||
| Sphaeroma terebrans has caused damage to docks, pilings, and boat hulls (Atwood 1920). Damage to plastic floats was extensive in southwest Florida and in Lake Pontchartrain LA (Davidson 2012). | |||||
| G170 | West Mississippi Sound | Ecological Impact | Habitat Change | ||
| In Lake Pontchartrain, Louisiana, S. terebrans extensively bored into Bald Cypress (Taxodium distichum) stumps, honeycombing them, and contributing to their decay and shoreline erosion (Wilkinson 2002; Davidson 2012). | |||||
| G170 | West Mississippi Sound | Economic Impact | Shipping/Boating | ||
| Sphaeroma terebrans has caused damage to docks, pilings, and boat hulls (Atwood 1920). Damage to plastic floats was extensive in southwest Florida and in Lake Pontchartrain LA (Davidson 2012). | |||||
| CAR-III | None | Economic Impact | Shipping/Boating | ||
| Damage to plastic floats was extensive in Colon, Panama (Davidson 2012). Boring by S. terebrans in plastic foam floats contirbute to pollution by plastic particles, with adverse consequences to marine foodwebs (Davidson 2012). | |||||
| CAR-II | None | Ecological Impact | Habitat Change | ||
| In caging experiments at Maya Walk and Maya Alcove, Belize, exclusion of S. terebrans resulted in much more extensive growth and complexity of mangrove roots (Davidson et al. 2016). | |||||
| EAS-III | None | Ecological Impact | Herbivory | ||
| Although Sphaeroma terebrans does not digest the roots of mangroves and other plants, the extensive boring of the roots of the mangrove Rhizophora mucronotata appears to limit the tree's distribution in the lower intertidal (Svavarsson et al. 2003). | |||||
| EAS-III | None | Ecological Impact | Habitat Change | ||
| Although Sphaeroma terebrans does not digest the roots of mangroves and other plants, the extensive boring of the roots of the mangrove Rhizophora mucronotata appears to contribute to the disintegration of collapse of trees in the lower intertidal. This may limit the extent of an important habitat for terrestrial amd marine biota (Svavarsson et al. 2003). | |||||
| NWP-3a | None | Ecological Impact | Herbivory | ||
| Although Sphaeroma terebrans does not digest the roots of mangroves and other plants, the extensive boring of the roots of the mangrove Rhizophora stylosa, and of the pneumatophores of Avicennia marina appears to limit the trees' distribution in the lower intertidal (Davidson et al. 2014). | |||||
| NWP-3a | None | Ecological Impact | Habitat Change | ||
| Although Sphaeroma terebrans does not digest the roots of mangroves and other plants, the extensive boring of the roots of the mangrove Rhizophora stylosa, and of the pneumatophores of Avicennia marina appears to limit the trees' distribution in the lower intertidal (Davidson et al. 2014). This may limit the extent of an important habitat for terrestrial amd marine biota. | |||||
| EA-III | None | Ecological Impact | Herbivory | ||
| Although Sphaeroma terebrans does not digest the roots of mangroves and other plants, the extensive boring of the roots of the mangrove Rhizophora mucronata appears to limit the trees' distribution in the lower intertidal (Davidson et al. 2014). | |||||
| EA-III | None | Ecological Impact | Habitat Change | ||
| Although Sphaeroma terebrans does not digest the roots of mangroves and other plants, the extensive boring of the roots of the mangrove Rhizophora mucronotata appears to limit the trees' distribution in the lower intertidal (Davidson et al. 2014). This may limit the extent of an important habitat for terrestrial and marine biota. | |||||
| FL | Florida | Ecological Impact | Habitat Change | ||
| Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 80-100% of mangrove roots infested, and 30-60% of roots severed (Conover and Reid 1975)., Extensive damage to roots of Red mangrove (Rhizophora mangle) was found with 70-80% of mangrove roots infested, and 20-40% of roots severed (Conover and Reid 1975). In caging experiments in Rookery Bay (Bear Creek and Environmental Learning Center) and Braden River, exclusion of S. terebrans resulted in much more extensive growth and complexity of mangrove roots (Davidson et al. 2016). Extensive burrowing was also found in tidal brackish and fresh marshes, in peat and roots of Juncus plants. Burrows were especially common on the scarps of receding marshes, and could be accelerating marsh retreat (Estevez 1994). Boring by S. terebrans in plastic foam floats contribute to pollution by plastic particles, with adverse consequences to marine foodwebs (Davidson 2012)., Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 80-100% of mangrove roots infested, and 60-100% of roots severed (Conover and Reid 1975). However, in experiments, R. mangle responds to boring activity by increasing the number of root endings in unparasitized roots, mostly those already buried in soil. Sphaeroma's boring activity could actually increase the stability of shorelines Simberloff et al. 1978; Ribi 1982)., Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 100% of mangrove roots infested, and 80-100% of roots severed (Rehm and Humm 1973; Conover and Reid 1975). In caging experiments in Rookery Bay (Environmental Learning Center, Bear Creek), exclusion of S. terebrans resulted in much more extensive growth and complexity of mangrove roots (Davidson et al. 2016)., Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 80-100% of mangrove roots infested, and 20-80% of roots severed (Conover and Reid 1975)., Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 100% of mangrove roots infested, and 90% of roots severed (Conover and Reid 1975)., Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 70% of mangrove roots infested, and 20% of roots severed (Conover and Reid 1975)., Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 100% of mangrove roots infested, and 80-100% of roots severed (Conover and Reid 1975)., Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 90-100% of mangrove roots infested, and 50-80% of roots severed (Conover and Reid 1975)., Extensive damage to roots of Red Mangrove (Rhizophora mangle) was found with 90% of mangrove roots infested, and 60% of roots severed (Conover and Reid 1975). | |||||
| FL | Florida | Economic Impact | Shipping/Boating | ||
| Sphaeroma terebrans has caused damage to docks, pilings, and boat hulls (Atwood 1920). Damage to plastic floats was extensive in southwest Florida and in Lake Pontchartrain LA (Davidson 2012). | |||||
Regional Distribution Map
| Bioregion | Region Name | Year | Invasion Status | Population Status | Vectors |
|---|---|---|---|---|---|
| AUS-XIII | None | 0 | Native | Established | |
| AUS-XII | None | 0 | Native | Established | |
| AUS-XI | None | 0 | Native | Established | |
| AUS-X | None | 0 | Native | Established | |
| AUS-XIX | None | 0 | Native | Established | |
| AUS-XIV | None | 0 | Native | Established | |
| AUS-I | None | 0 | Native | Established | |
| AUS-II | None | 0 | Native | Established | |
| AUS-III | None | 0 | Native | Established | |
| EAS-VIII | None | 0 | Native | Established | |
| EAS-III | None | 0 | Native | Established | |
| EA-III | None | 0 | Native | Established | |
| SP-I | None | 0 | Native | Established | |
| EAS-III | None | 0 | Native | Established | |
| CIO-I | None | 0 | Native | Established | |
| CIO-II | None | 0 | Native | Established | |
| CIO-III | None | 0 | Native | Established | |
| CIO-IV | None | 0 | Native | Established | |
| EAS-VI | None | 0 | Native | Established | |
| EAS-VII | None | 0 | Native | Established | |
| EAS-II | None | 0 | Native | Established | |
| EAS-I | None | 0 | Native | Established | |
| EAS-IV | None | 0 | Native | Established | |
| NA-ET3 | Cape Cod to Cape Hatteras | 1962 | Non-native | Unknown | |
| CAR-VII | Cape Hatteras to Mid-East Florida | 1881 | Non-native | Established | |
| CAR-I | Northern Yucatan, Gulf of Mexico, Florida Straits, to Middle Eastern Florida | 1906 | Non-native | Established | |
| CAR-II | None | 1980 | Non-native | Established | |
| CAR-V | None | 1994 | Non-native | Established | |
| CAR-III | None | 1985 | Non-native | Established | |
| IP-1 | None | 0 | Native | Established | |
| EA-IV | None | 0 | Native | Established | |
| WA-V | None | 0 | Crypogenic | Established | |
| WA-II | None | 1977 | Non-native | Established | |
| S190 | Indian River | 1973 | Non-native | Established | |
| G130 | Pensacola Bay | 1952 | Non-native | Established | |
| S180 | St. Johns River | 1897 | Non-native | Established | |
| G070 | Tampa Bay | 1910 | Non-native | Established | |
| M130 | Chesapeake Bay | 1962 | Non-native | Unknown | |
| G250 | Sabine Lake | 1945 | Non-native | Established | |
| S200 | Biscayne Bay | 1975 | Non-native | Established | |
| SA-II | None | 1866 | Non-native | Established | |
| G170 | West Mississippi Sound | 1916 | Non-native | Established | |
| G030 | North Ten Thousand Islands | 1973 | Non-native | Established | |
| NWP-3a | None | 0 | Native | Established | |
| G045 | _CDA_G045 (Big Cypress Swamp) | 1972 | Non-native | Established | |
| S110 | Broad River | 1891 | Non-native | Established | |
| S183 | _CDA_S183 (Daytona-St. Augustine) | 1923 | Non-native | Established | |
| G190 | Mississippi River | 1923 | Non-native | Established | |
| S175 | _CDA_S175 (Nassau) | 1939 | Non-native | Established | |
| S080 | Charleston Harbor | 1943 | Non-native | Established | |
| S196 | _CDA_S196 (Cape Canaveral) | 1975 | Non-native | Established | |
| G010 | Florida Bay | 1974 | Non-native | Established | |
| G020 | South Ten Thousand Islands | 1973 | Non-native | Established | |
| G050 | Charlotte Harbor | 1974 | Non-native | Established | |
| G056 | _CDA_G056 (Sarasota Bay) | 1975 | Non-native | Established | |
| G074 | _CDA_G074 (Crystal-Pithlachascotee) | 1975 | Non-native | Established | |
| S206 | _CDA_S206 (Vero Beach) | 1945 | Non-native | Established | |
| G270 | Brazos River | 1945 | Non-native | Established | |
| NWP-2 | None | 0 | Native | Established | |
| EA-V | None | 0 | Native | Established | |
| WA-IV | None | 1911 | Non-native | Established | |
| WA-III | None | 1889 | Non-native | Established | |
| S140 | St. Catherines/Sapelo Sounds | 1978 | Non-native | Established | |
| EA-III | None | 0 | Native | Established | |
| PAN_CAR | Panama Caribbean Coast | 2012 | Non-native | Established | |
| SA-III | None | 0 | Non-native | Established | |
| SP-VII | None | 0 | Native | Established |
Occurrence Map
| OCC_ID | Author | Year | Date | Locality | Status | Latitude | Longitude |
|---|
References
Atlas of Living Australia 2013-2016 Atlas of Living Australia. <missing URL>Atwood, W. G. (1922) Marine borers, Proceedings of the American Society of Civil Engineers 48(6): 1408-1424
Baldwin, Andy; Leason, Diane (2016) Potential Ecological impacts of Emerald Ash Borer on Maryland's Eastern Shore, In: None(Eds.) None. , <missing place>. Pp. <missing location>
Banta, W. C. 1996 09.522 Field and lab methods in water quality analysis- Fall 1996.. <missing URL>
Baratti, M.; Filippelli, M.; Messana, G. (2011) Complex genetic patterns in the mangrove wood-borer Sphaeroma terebrans Bate, 1866 (Isopoda, Crustacea, Sphaeromatidae) generated by shoreline topography and rafting dispersal, Journal of Experimental Marine Biology and Ecology 398: 73-82
Baratti, Mariella; Goti, Emanuele; Messana, Giuseppe (2005) High level of genetic differentiation in the marine isopod Sphaeroma terebrans (Crustacea Isopoda Sphaeromatidae) as inferred by mitochdrial DNA analysis., Journal of Experimental Marine Biology and Ecology 315: 225-234
Becker, Gunther (1971) On the biology, physiology, and ecology of marine wood-boring crustaceans., In: Gareth Jones, E. B.//Eltringham, S. K.(Eds.) Marine borers, fungi, and fouling organisms of wood.. , Brussels. Pp. 303-326
Brooks, R. Allen (2004) Discovery of Sphaeroma terebrans, a wood-boring isopod, in the red mangrove, Rhizophora mangle, habitat of northern Florida Bay., Ambio 33(3): 171-173
Brooks, R. Allen; Bell, Susan S. (2005) The distribution and abundance of Sphaeroma terebrans, a wood boring isopod of red mangrove (Rhizophora mangle) habitat within Tampa Bay., Bulletin of Marine Science 76(1): 27-46
Bruce, Niel L.; Wong, Helen P.-S. (2015) An overview of the marine Isopoda (Crustacea) of Singapore, Raffles Bulletin of Zoology Supplement No. 31:: 152-168
Carlton, James T. (2000) Marine bioinvasions ecology in the 21st century, In: Pederson, Judith(Eds.) Marine Bioinvasions. , Cambridge MA. Pp. 6-23
Carlton, James T.; Iverson, Ernest W. (1981) Biogeography and natural history of Sphaeroma walkeri Stebbing (Crustacea: Isopoda) and its introduction to San Diego Bay, California, Journal of Natural History 15: 31-48
Carlton, James T.; Ruckelshaus, Mary H. (1997) Nonindigenous marine invertebrates and algae of Florida, In: Simberloff, Daniel, Schmitz, Don C., Brown, Tom C.(Eds.) Strangers in Paradise: Impact and Management of Nonindigenous Species in Florida. , Washington, D.C.. Pp. 187-201
Clamp, John C. (2006) Redescription of Lagenophrys cochinensis (Ciliophora, Peritrichia, Lagenophryidae), anectosymbiont of marine isopods, including new information on morphology, geographic distribution, and intraspecific variation., Journal of Eukaryotic Microbiology 53(1): 58-64
Conover, David O.; Reid, George K. (1975) Distribution of the boring isopod Sphaeroma terebrans in Florida, Florida Scientist 38(2): 65-72
Davidson, Timothy M. (2012) Boring crustaceans damage polystyrene floats under docks polluting marine waters with microplastic, Marine Pollution Bulletin 64: 1821-1828
Davidson, Timothy M.; de Rivera, Catherine E.; Hsieh, Hwey-Lian (2014) Damage and alteration of mangroves inhabited by a marine wood-borer, Marine Ecology Progress Series 516: 177-185
Estevez, Ernest D. (1994) Inhabitation of tidal salt marshes by the estuarine wood-boring isopod Sphaeroma terebrans in Florida., In: Thompson, M.-F. and Nagabhushanam, R.(Eds.) Recent developments in biofouling control. , New Delhi. Pp. 97-105
Faasse, Marco (2012) The exotic isopod Synidotea in the Netherlands and Europe, A Japanese or American invasion (Pancrustacea: Isopoda)?, Nederlandse Faunistiche Mededelingen 108: 103-106
Harrel, Richard C.; Ashcraft, Jimmy; Howard, Randall; Patterson, Larry (1976) Stress and community structure of macrobenthos in a Gulf Coast riverine estuary., Contributions in Marine Science 20: 69-81
Harrison, K.; Ellis, J. P. (1991) The genera of the Sphaeromatidae (Crustacea: Isopoda): a key and distribution list, Invertebrate Taxonomy 5: 915-952
Harrison, K.; Holdich, D. M. (1984) Hemibranchiate sphaeromatids (Crustacea: Isopoda) from Queensland, Australia, with a world-wide review of the genera discussed, Zoological Journal of the Linnean Society 81: 275-387
Herms, Daniel A. McCullough, Deborah G. (2014) Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management, Annual Review of Entomology 59: 13–30
Hossain, M. Belal; Bamber, Roger N. (2013) New record of a wood-boring isopod, Sphaeroma terebrans (Crustacea: Sphaeromatidae) from Sungai Brunei estuary, Brunei Darussalam, Marine Biodiversity Records 6: e18
Karatayev AY, Burlakova LE, Karatayev VA, Boltovskoy D (2010) Limnoperna fortunei versus Dreissena polymorpha: population densities and benthic community impacts of two invasive freshwater bivalves, Journal of Shellfish Research 29(4): 975–984
https://doi.org/10.2983/0730-8000\(2007\)26[205:TIBDPA]2.0.CO;2
Kensley, Brian (1978) <missing title>, Trustees of the South African Musuem, Cape Town. Pp. <missing location>
Kensley, Brian; Nelson, Walter G.; Schotte, Marilyn (1995) Marine isopod biodiversity of the Indian River Lagoon, Florida, Bulletin of Marine Science 57(1): 136-142
Kensley, Brian; Schotte, Marilyn (1989) <missing title>, Smithsonian Institution Press, Washington, D.C.. Pp. <missing location>
Kensley, Brian; Schotte, Marilyn (1999) New records of isopods from the Indian River Lagoon, Florida (Crustacea: Peracarida)., Proceedings of the Biological Society of Washington 112(4): 695-713
Keppel, Erica; , Keith, Inti; Ruiz, Gregory M.; Carlton, James T. (2019) New records of native and non-indigenous polychaetes (Annelida: Polychaeta) in the Galapagos Islands, Aquatic Invasions 14(1): 59-84
Kramp, P. L. (1969) The hydromedusae of the Pacific and Indian Oceans, Dana Reports 72: 1-200
Kussakin, Oleg G.; Malyutina, Marina V. (1993) Sphaeromatidae (Crustacea: Isopoda: Flabellifera) from the South China Sea., Invertebrate Taxonomy 7: 1167-203
Liu, Wenliang; Liang, Xiaoli ; Zhu, Xiaojing (2015) A new record and mitochondrial identification of Synidotea laticauda Benedict, 1897 (Crustacea: Isopoda: Valvifera: Idoteidae) from the Yangtze Estuary, China, Zootaxa 4294: 371-380
Lomonaco, Cecilia; Santos, Andre S.; Christoffersen, Martin l. (2011) Effects of local hydrodynamic regime on the individual’s size in intertidal Sabellaria (Annelida: Polychaeta: Sabellariidae) and associated fauna at Cabo Branco beach, north-east Brazil, Marine Biodiversity Records 4(e76): Published online
doi:10.1017/S1755267211000807;
Mead, A.; Carlton, J. T.; Griffiths, C. L. Rius, M. (2011b) Introduced and cryptogenic marine and estuarine species of South Africa, Journal of Natural History 39-40: 2463-2524
Miller, Milton A. (1968) Isopoda and Tanaidacea from buoys in coastal waters of the continental United States, Hawaii, and the Bahamas (Crustacea), Proceedings of the United States National Museum 125(3652): 1-53
Montalvo-Urgel, Hugo; Sánchez, Alberto J.; Florido, Rosa; Macossay-Cortez, Alberto A. (2010) [List of crustaceans distributed in submerged woody debris in the tropical wetlands of Pantanos de Centla, southern Gulf of Mexico], Revista Mexicana de Biodiversidad 81: S121- S131
Muirhead, Jim R.; Leung, Brian; van Overdijk, Colin; Kelly, David W.; Nandakumar, Kanavillil; Marchant, Kenneth R.; MacIsaac, Hugh J. (2006) Modelling local and long-distance dispersal of invasive emerald ash borer Agrilus planipennis (Coleoptera) in North America, Diversity and Distributions 12: 71–79
Nair, N. Balakrishnan (1984) The problem of marine timber destroying organisms along the Indian coast, Proceedings of the Indian Academy of Sciences 93(3): 203-223
Perry, Diane M.; Brusca, Richard C. (1989) Effects of the root-boring Sphaeroma peruvianum on red mangrove forests., Marine Ecology Progress Series 57: 287-292
Pillai, N. Krishna (1967) Proceedings of the symposium on Crustacea, Pt. V Marine Biological Association of India, <missing place>. Pp. 1274-1283
Pires, Ana Maria Setubal (1982) [Sphaeromatidae (Isopoda:Flabellifera) of the intertidal and shallow depths of the states of Sao Paulo and Rio de Janeiro, Boletim do Instituto Oceanográfico 31(2): 43-55
Rehm, Andrew; Humm, Harold J. (1973) Sphaeroma terebrans: a threat to the mangroves of southwestern Florida., Science 182: 173-174
Ribi, Georg (1982) Differential colonization of roots of Rhizophora mangle by the wood boring isopod Sphaeroma terebrans as a mechanism to increase root density., Pubblicazioni della Stazione Zoologica di Napoli I: Marine Ecology 3(1): 13-19
Richardson, Harriet (1897) Description of a new species of Sphaeroma., Proceedings of the Biological Society of Washington 11: 105-107
Richardson, Harriet (1905) A monograph on the isopods of North America, United States National Museum Bulletin 54: 1-727
Rotramel, George L. (1975) Observations on the commensal relations of Iais californica (Richardson, 1904) and Sphaeroma quoyanum H. Milne Edwards, 1840 (Isopoda), Crustaceana 28(3): 247-256
Schultz, George A. (1978) Four marine isopod crustaceans from St. Catherines Island with a list of other species from Georgia, Georgia Journal of Science 36: 1-17
Si, Aung; Bellwood, O; Alexander, C. G. (2002) Evidence for filter-feeding by the wood-boring isopod, Sphaeroma terebrans (Crustacea: Peracarida), Journal of Zoology 256: 463-471
Simberloff, Daniel; Brown. Becky J.; Lowrie, Stuart (1978) Isopod and insect borers may benefit Florida mangroves., Science 201: 630-632
Soors, Jan; Faasse, Marco; Stevens, Maarten; Verbessem, Ingrid; De Regge, Nico;Van den Bergh, Ericia (2010) New crustacean invaders in the Schelde estuary (Belgium), Belgian Journal of Zoology 140: 3-10
Thiel, M. (1999) Host and population demographics of the ascidian-dwelling amphipod Leucothoe spinicarpa: indication for extended parental care and advanced social behavior, Journal of Natural History 33: 193-206
U.S. National Museum of Natural History 2002-2021 Invertebrate Zoology Collections Database. http://collections.nmnh.si.edu/search/iz/
Van Name, Willard G. (1936) The American land and fresh-water isopod Crustacea, Bulletin of the American Museum of Natural History 71: 1-535
Villalobos, Carlos R; Cruz, Gastavo A.; Cruz, Rafael A.. (1985) Notas sobre la biologia de Sphaeroma terebrans Bate. 1866 (Sphaeromatidae: Isopoda) en el manglar de Pochote, Provincia de Puntarenas, Costa Rica., Brenesia 24: 287-296
Wurtz, Charles B.; Roback, Selwyn S. (1955) Invertebrate fauna of some Gulf coast rivers, Proceedings of the Academy of Natural Sciences of Philadelphia 107: 167-206