Invasion History
First Non-native North American Tidal Record: 1948First Non-native West Coast Tidal Record: 1948
First Non-native East/Gulf Coast Tidal Record: 1978
General Invasion History:
Lernaea cyprinacea was first described from Europe in 1745 under a trinomial name, and was redescribed by Linnaeus in 1758 (Kabata 1979). This freshwater parasitic copepod infects a wide range of teleost fish (100+ species) and also amphibians (Hoffman 1967; Kabata 1979). Consequently, it has been widely spread throughout most of the world with ornamental and stocked fishes. Stocks and escapes of Carassius auratus (Goldfish) and Cyprinus carpio (Common Carp) may be the most likely vectors (Hoffman 1967; Tidd 1934). It is probably native to Asia, including Japan, and has been introduced to mainland Europe, the British Isles, Africa, Australia, North America, and South America (Hall 1983; Hoffman 1967; Kabata 1979; Kennedy 1993).
Lernaea cyprinacea was first reported (as L. carassii) in North America from goldfish farms in Ohio (1929) and Indiana (Tidd 1934). Wilson (1918) mentioned it in a global survey of the genus, but did not report it from this continent. Tidd (1934) notes: 'If Lernaea carassii were a species native to the United States, it seems strange that it could have so long escaped the attention of fanciers of ornamental fish and fisheries biologists. While at present I have records for the occurrence of the 'anchor parasite' in Ohio and Indiana only, I would not be surprised to find that it already has a wide distribution range'. Even at this early date, L. cyprinacea was already known from nine species of fishes (Tidd 1934). Hoffman (1967) cited records from 11 states, including many midwestern states, and Maryland, California, Washington, and Arizona. A search of recent citations adds 10 states to this list, so L. cyprinacea is probably found in most of North America. Records from estuaries are spotty, probably because few surveys of freshwater fish parasites have been conducted in tidal waters. This parasite is likely to occur in most tidal fresh waters in temperate North America.
North American Invasion History:
Invasion History on the West Coast:
In the Columbia River system, Lernaea cyprinacea was found in Lake Oswego, Oregon, adjacent to the tidal Willamette River, above Portland, on Oncorhychus tshawytscha (Chinook Salmon) and in Tigard, Oregon, in a small private pond, on Oncorhychus mykiss (Rainbow Trout) (both in 1948, Uzman and Rayner 1958). In the non-tidal watershed, it was found in the lower Yakima River on four species of introduced fishes and four species of native fishes in 1957 (Uzman and Rayner 1958).
In the San Francisco Bay estuary, L. cyprinacea was collected at the Fish screen facility in Tracy, California in the Sacramento-San Joaquin Delta during a survey of fish parasites in 1973. It was found on introduced Ameiurus catus (White Catfish) and four native fishes Mylopharodon conocephalus (Hardhead); Orthodon microlepidotus (Sacramento Blackfish); Pogonichthys macrolepidotus (Splittail); and Ptychocheilus grandis (Sacramento Squawfish) (Hensley and Nahhas 1975).
Invasion History on the East Coast:
Lernaea cyprinacea was listed as a fish parasite in the Coastal Zone of South Carolina, from White Catfish (Amieurus catus) (Lawler 1978). It is probably widespread in fresh and low-salinity zones of many East and Gulf Coast estuaries. We did not find records from tidal waters of Chesapeake Bay, but it was abundant in a pond in College Park, Maryland (Haley and Winn 1959) and in the Susquehanna River in central Pennsylvania (Deutsch 1977; 1978; and 1984), affecting at least 18 species of native and introduced fishes.
Invasion History Elsewhere in the World:
Lernaea cyprinacea may have been introduced to Western Europe with Common Carp (Cyprinus carpio) in Roman times, or later with Goldfish (Carassius auratus). However, it was not reported from England until 1960 (Kennedy 1993) and in the Ebro River system, Spain it was first reported in 1995 (Oscoz et al. 2009), so it may still be extending its range.
In Africa, it was found in Lake Victoria by 1956, and was widespread in South Africa by the 1990s (Robinson and Avenant-Oldewage 1996). It was present in Australia by the 1970s (Hall 1983; Marina et al. 2008) and Brazil by the 1980s (Maghalaes 2006). In each case, it spread rapidly onto native fishes.
Description
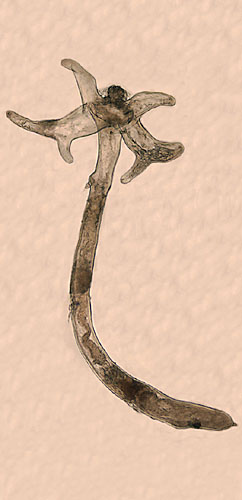
Lernaea cyprinacea is an ectoparasitic copepod, whose adult females normally parasitize freshwater fishes, but occasionally attack amphibians. The free-swimming opepodites, the adult male, and the pre-attachment female are somewhat like that of a cyclopoid copepod. The adult female metamorphoses after attachment to a host and becomes highly modified, with the cephalothorax expanded laterally into 2 pairs of horns (part of its holdfast) and a wormlike, elongated thoracic portion, with 3 pairs of vestigial swimming legs along its length. At the end of the thorax is a pregenital swelling, anterior to the oviducts, where paired egg-sacs are attached. There is a short, conical abdomen, usually at an angle to the rest of the body, ending in pair of hair-like caudal rami. The lateral horns and long body give rise to the name 'anchor-worm'. The horns are derived from the maxillipeds and 1st swimming legs. They are quite variable in shape, with the dorsal pair much larger than the ventral, divided into two branches, some distance from their bases. The ventral pair is slender, and usually simple. The overall length of the attached female is 10-20 mm. Description based on: Tidd 1930; Demaree 1967; Kabata 1979; and Robinson and Avenant-Oldewage 1996.
Pre-attachment females and males are similar in general body shape. The cephalothorax is shield-shaped, with a rounded anterior and parallel sides. A U-shaped groove divides the head from the 1st leg-bearing segment. There are five thoracic segments, and a urosome consisting of 4 segments. Overall, the female body tapers uniformly – the 1st urosome (genital) segment is about the same width as the 5th thoracic segment. The small, tapered, caudal rami have small lateral setae and single long terminal setae. The antennules (1st antennae) are uniramous, with 6 segments. Pre-attachment females are 1.2-1.4 mm long (Kabata 1979).
Male L. cyprinacea have a narrower urosome and genital segment than the female. The antennules have 8 segments. They are smaller than the pre-metamorphosis females, about 1.1 mm in size (Grabda 1963; Kabata 1979). After hatching, this copepod goes through 3 nauplius stages, and 5 copedite stages, before molting into a 'cyclopoid' adult stage (Grabda 1963). This is the final development stage for males, but females go through a radical transformation into a wormlike form, after attachment to a host, usually a fish, but sometimes a frog (Grabda 1963; Kabata 1979).
The taxonomy of this copepod is complex, because the morphology of the attachment structures varies greatly in the adult female according to the site of attachment, the shape and behavior of the host, and the surrounding environment (Kabata 1979).
Taxonomy
Taxonomic Tree
| Kingdom: | Animalia | |
| Phylum: | Arthropoda | |
| Subphylum: | Crustacea | |
| Class: | Maxillopoda | |
| Subclass: | Copepoda | |
| Order: | Caligoida | |
| Family: | Lernaeidae | |
| Genus: | Lernaea | |
| Species: | cyprinacea |
Synonyms
Lernaea elegans (None, None)
Lernaea esocina (Burmeister, 1835)
Lernaea ranae (Stunkard & Cable, 1931)
Lernaea tentaculis quattor (Linnaeus, 1758)
Lernaeocera cyprinacea (Blainville, 1822)
Lernaeocera gasterostei (Bruhl, 1860)
Potentially Misidentified Species
On Micropterus spp., North Carolina, Louisiana
Lernaea cruciata
On Ambloplites rupestris, Micropterus spp. (Lake Erie), Lepomis gibbosus, Morone americana; Massachusetts
Ecology
General:
Lernaea cyprinacea is a parasitic copepod, in which the adult female, after fertilization, becomes a worm-like parasite, permanently attached to a fish or amphibian host. The transformed female produces many batches of attached, paired egg sacs, which hatch into non-feeding nauplius larvae. After three nauplius stages, the animal molts into the copepodite I stage. This stage, and successive stages II-V attach temporarily to fish hosts and feed from them. However, they are capable of swimming and moving from one host to another. Copepodite V molts into a free-swimming cyclopoid (adult) stage. Females attach to the surface of a host (not clear if this happens before or after mating), and mating occurs. The fertilized female then undergoes its transformation, penetrating the skin of a host and anchoring in the muscle tissue (Grabda 1963; Kabata 1979; Wilson 1918).
Lernaea cyprinacea is an ectoparasite with an extraordinary range of hosts, primarily fishes (more than 100 species), but also frogs (Bullfrogs- Lithobates catesbiana, Rana pipiens, Rana clamitans) and other species (Hofmann 1968; Shields and Tidd 1968; Kabata 1978). We have no records of this parasite from brackish waters. It has infected the euryhaline fish Fundulus heteroclitus (Mummichog) under experimental conditions, and tolerated salinities up to 12 PSU, but eggs did not develop at salinities above 3 .5 PSU (Shields and Sperber 1974).
Food:
Fishes (many species); Frogs (Rana sp.)
Trophic Status:
Parasite
ParasHabitats
| General Habitat | Fresh (nontidal) Marsh | None |
| General Habitat | Grass Bed | None |
| General Habitat | Coarse Woody Debris | None |
| General Habitat | Nontidal Freshwater | None |
| General Habitat | Tidal Fresh Marsh | None |
| General Habitat | Unstructured Bottom | None |
| Salinity Range | Limnetic | 0-0.5 PSU |
| Salinity Range | Oligohaline | 0.5-5 PSU |
| Tidal Range | Subtidal | None |
| Vertical Habitat | Nektonic | None |
Tolerances and Life History Parameters
| Minimum Temperature (ºC) | 0 | Based on geographic range |
| Maximum Temperature (ºC) | 36 | Highest temperature tested (Shields and Tidd 1968). |
| Minimum Salinity (‰) | 0 | A freshwater species. |
| Maximum Salinity (‰) | 13 | For adult parasites attached to the euryhaline fish Fundulus heteroclitus (Mummichog) (Shields and Sperber (1979). |
| Minimum Reproductive Temperature | 20 | Experimental (Shields and Tidd 1968 |
| Maximum Reproductive Temperature | 36 | Highest temperature tested (Shields and Tidd 1968). |
| Minimum Reproductive Salinity | 0 | A freshwater species. |
| Maximum Reproductive Salinity | 3.5 | For adult parasites attached to the euryhaline fish Fundulus heteroclitus (Mummichog) (Shields and Sperber (1979). |
| Broad Temperature Range | None | Cold temperate-Tropical |
| Broad Salinity Range | None | Nontidal Limnetic-Tidal Limnetic |
General Impacts
Economic ImpactsFisheries: Lernaea cyprinacea is now distributed throughout the temperate and tropical regions of the world, and is known from 100+species of fishes (Hoffman 1967; Hoffman and Schubert 1984; Kabata 1979). In cultured fish populations, it can be devastating, causing extensive mortality of fishes such as goldfish and carp, as well as unsightly lesions (Khalifa and Post 1976; Tidd 1934). High densities of fish in rearing ponds are likely to greatly favor this and other parasites (Wilson 1918).
While the occurrence of L. cyprinacea in wild fish populations has been frequently studied, its effects on population density, biomass, and structure are poorly known (Eisen 1983). Consequently, its effects on fisheries cannot be quantified. However, high rates of parasitism by L. cyprinacea are likely to detract from the perceived food and sporting qualities of freshwater fish.
Ecological Impacts
Parasitism: The occurrence, seasonal abundance, and host preference of Lernaea cyprinacea has been widely studied throughout its range (e.g. Adams 1984; Deutsch 1977; Deutsch 1978; Deutsch 1989; Haley and Winn 1959; Marcogliese 1991; Timmons and Hemstreet 1980). Although L. cyprinacea shows some preference for cyprinids and related families (e.g. Haley and Winn 1959), it is known to feed on more than 100 fish species, as well as the tadpoles of frogs and salamanders (Hoffman 1967; Kabata 1979; Tidd and Shields 1963). Infection of tadpoles with L. cyprinacea have been seen in the field as well as in the laboratory.
In experiments, copepodites of Lernaea cyprinacea favored the fins of Carassius auratus and the mouth and branchial chamber of tadpoles of Rana clamitans and Rana pipiens (Shields and Tidd 1974). However, in field surveys, gill infestations by copepodites are common (e.g., Deutsch 1978; Haley and Winn 1959). Heavy infestations of L. cyprinacea copepodites on the fins can result in fatal inflammation (Shields and Tidd 1974). Heavy infections of copepodites are especially damaging to the gill tissues, causing irritation and necrosis, and can kill the fish through lack of oxygen (Khalifa and Post 1976).
Adult L. cyprinacea can attack any part of the body surface of a fish, but the fins and gills are the most frequent sites of attachment (Deutsch 1978; Haley and Winn 1959; Timmons and Hemstreet 1980). The attached transformed adult female penetrates the skin of fishes and becomes deeply embedded in the host tissue, causing lesions which become hemorrhagic and eventually, necrotic. Muscle bundles near the site of penetration are also destroyed; penetration of the cranium and the walls of the intestine can also occur. The host response includes inflammation and occasionally the development of tumor-like growths around the site of penetration. Secondary bacterial and fungal infections around the lesion are also common (Khalifa and Post 1976). In tadpoles, the anchor of the parasite can affect the kidneys, liver, and spinal cord, as well as the muscle. However, the skin does not show the inflammation and abnormal growth responses characteristic of L. cyprinacea infestation in fishes (Tidd and Shields 1963). Parasitism by L. cyprinacea frequently causes mortality in fishes and tadpoles, particularly in smaller individuals. This may vary with the site of penetration, among other factors (Khalifa and Post 1976). However, parasitized fish are capable of rejecting adult parasites, even after host penetration. The host's ability to do this is highly variable, and may depend on immunological history and overall health (Shields and Goode 1978).
While L. cyprinacea has been extensively studied in terms of prevalence and pathology, and it is known as a serious pest in aquaculture situations, its impact on the density, biomass, and age-structure of natural fish populations is not well understood. Parasitism by L. cyprinacea did not influence mortality due to predation in parasitized Pimephales promelas (Fathead Minnow) exposed to Walleye (Sander vitreus) and Northern Pike (Esox lucius) (Vaughan and Coble 1975). In many natural populations, while a high percentage of fish are infested, most have only one parasite. Eisen (1983) has modeled the mortality as a function of parasite prevalence, parasites per fish, and the proportion of a fish's surface which is 'critical', where penetration is likely to be lethal. He argues that the parasite population is regulated by the mortality of the host, but that parasite density does not regulate the host population (Eisen 1983). However, empirical studies of natural populations of the host and parasite have not been conducted.
Regional Distribution Map
| Bioregion | Region Name | Year | Invasion Status | Population Status | Vectors |
|---|---|---|---|---|---|
| GL-II | Lake Erie | 1931 | Non-native | Established | |
| P090 | San Francisco Bay | 1973 | Non-native | Established | |
| P260 | Columbia River | 1948 | Non-native | Established | |
| S060 | Winyah Bay | 1978 | Non-native | Established | |
| S070 | North/South Santee Rivers | 1978 | Non-native | Established | |
| S080 | Charleston Harbor | 1978 | Non-native | Established | |
| S090 | Stono/North Edisto Rivers | 1978 | Non-native | Established | |
| S110 | Broad River | 1978 | Non-native | Established | |
| S100 | St. Helena Sound | 1978 | Non-native | Established | |
| S120 | Savannah River | 1978 | Non-native | Established | |
| NEP-V | Northern California to Mid Channel Islands | 1973 | Non-native | Established | |
| NEP-IV | Puget Sound to Northern California | 1948 | Non-native | Established | |
| CAR-VII | Cape Hatteras to Mid-East Florida | 1978 | Non-native | Established |
Occurrence Map
| OCC_ID | Author | Year | Date | Locality | Status | Latitude | Longitude |
|---|---|---|---|---|---|---|---|
| 31778 | OSPR - Historic Data | 1973 | 9999-01-01 | Fish screen facility, Tracy | Non-native | 37.7965 | -121.5849 |
References
Adams, Ann M. (1984) Infestation of Fundulus kansae (Garman) (Pisces: Cyprinodontidae) by the copepod Lernaea cyprinacea Linnaeus, 1758, in the South Platte River, Nebraska., American Midland Naturalist 112: 131-137Burris, Kenneth Wayne; Miller, Grover C. (1972) Parasitic copepods of some freshwater fishes from North Carolina, Journal of the Elisha Mitchell Scientific Society 88: 18-20
de Magalhães, André Lincoln Barroso (2006) First record of lernaeosis in a native fish species from a natural environment in Minas Gerais state, Brazil, Pan-American Journal of Aquatic Sciences 1(1): 8-10
Dechtiar, Alex O. (1972) New parasite records for Lake Erie fish., Great Lakes Fisheries Commission Technical Report 27: 1-20
Demaree, Richard S., Jr. (1967) Ecology and external morphology of Lernaea cyprinacea., American Midland Naturalist 78: 416-427
Deutsch, Wiliam G. (1978) Lernaea cyprinacea on two catostomid fishes., Proceedings of the Pennsylvania Academy of Science 52: 57-59
Deutsch, William G. (1977) Fish parasites from the Susquehanna River in Pennsylvania, with new host records, Proceedings of the Pennsylvania Academy of Science 51: 122-124
Deutsch, William G. (1984) Parasites of Susquehanna River (Pennsylvania) Muskellunge, Journal of the Pennsylvania Academy of Science 63(1): 25-27
Eisen, Stanley (1983) An alternative model based on random distributions for density-dependent regulation in host-parasitic systems., American Midland Naturalist 109: 230-239
Grabda, Jadwiga (1963) Life cycle and morphogenesis of Lernaea cyprinacea L., Parasitologica Polonica 11: 169-199
Haley, A. James; Winn, Howard E. (1959) Observations on a lernaean parasite of freshwater fishes, Transactions of the American Fisheries Society 88(1): 128-129
Hall, D. N. (1983) Occurrence of the copepod parasite Lernaea cyprinacea L., on the Australian grayling, Prototroctes maraena Gunther., Proceedings of the Royal Society of Victoria 95(3): 273-274
Hensley, Gary H., Nahhas, F.M. (1975) Parasites of fishes from the Sacramento-San Joaquin Delta, California., California Fish and Game 61(4): 201-208
Hoeksema; Bert W.; Samimi-Namin, Kaveh McFadden, Catherine S. ; Rocha, Rosana M.; van Ofwegen, Leen P. ; Hiemstra,, Auke-Florian; Vermeij. Mark J. A. (2023) Non-native coral species dominate the fouling community on a semi-submersible platform in the southern Caribbean, Marine Pollution Bulletin 194(115353): Published online
Hoffman, Glenn L. (1967) Parasites of North American freshwater fishes, In: (Eds.) . , Berkeley. Pp. <missing location>
Hoffman, Glenn, L.; Schubert, Gottfried (1984) Distribution, Biology, and Management of Exotic Fishes., Johns Hopkins University Press, Baltimore, MD. Pp. 233-261
Idris, H.B.; Amba, M.A. (2011) A note on Lernaea cyprinacea parasitizing the cultured marble goby Oxyeleotris marmorata and their control with salinity modification, Advances in Environmental Biology 5(5): 817-820
Kabata, Z. (1979) <missing title>, Ray Society, London. Pp. <missing location>
Kennedy, C. R. (1993) Introductions, spread, and colonization of new localities by fish helminth and crustacean parasites in the British Isles: A perspective and appraisal, Journal of Fish Biology 43: 287-301
Khalifa A.; Post, George (1976) Histopathological effect of Lernaea cyprinacea (a copepod parasite) on fish, Progressive Fish Culturist 38: 110-113
Lawler, Adrian R. (1978) An annotated checklist of the biota of the coastal zone of South Carolina, University of South Carolina Press, Columbia. Pp. 309-345
Marcogliese, David J. (1991) Seasonal occurrence of Lernaea cyprinacea on fish in Belews Lake, North Carolina., Journal of Parasitology 77: 326-327
Marina, H.; Beatty, S. J.; Morgan, D. L.; Doupé, R. G.; Lymbery, A. J. (2008) An introduced parasite, Lernaea cyprinacea L., found on native freshwater fishes in the south west of Western Australia, Journal of the Royal Society of Western Australia 91: 149-153
Oscoz, Javier; Tomás, Pedro; Durán, Concha (2009) Review and new records of non-indigenous freshwater invertebrates in the Ebro River basin (Northeast Spain), Aquatic Invasions 5(3): 263-284
Robinson, Jenny; Avenant-Oldewage, A. (1996) Aspects of the morphology of the parasitic copepod Lernaea cyprinacea Linnaeus, 1758 and notes on its distribution in Africa, Crustaceana 69(5): 610-626
Shariff, M.; Sommerville, C. (1989) Morphometrics of the larval stages of Lernaea polymorpha Yu and L. cyprinacea Linnaeus (Copepoda), Crustaceana 57(2): 134-139
Shields, Robert J. (1968) Experimental infestation of Fundulus heteroclitus (L.) (Pisces) by Lernaea cyprinacea L. (Copepoda), Crustacean 15: 111-112
Shields, Robert J., Sperber, Robert G. (1974) Osmotic regulation of Lernaea cyprinacea L. (Copepoda), Crustacean 26(2): 157-172
Shields, Robert J., Tidd, Wilbur M. (1968) Site selection on hosts by copepodids of Lernaea cyprinacea L. (Copepoda)., Crustacean 1: 88-94
Shields, Robert J., Tidd, Wilbur M. (1974) Effect of temperature on the development of larval and transformed females of Lernaea cyprinadea L. (Lernaeidae), Crustacean 27: 225-230
Tidd, Wilbur M. (1930) A new species of Lernaea (parasitic Copepoda) from the goldfish., Ohio Journal of Science 33: 465-470
Tidd, Wilbur M.; Shields, Robert J. (1963) Tissue damage inflicted by Lernaea cyprinacea Linnaeus, a copepod parasitic on tadpoles, The Journal of Parasitology 49(4): 693-696
Tidd, Wilbur, M, (1934) Recent infestations of goldfish and carp by the 'anchor parasite,' Lernaea cyprinacea., Transactions of the American Fisheries Society 64: 176-180
U.S. National Parasite Collection Unit, United States Department of Agriculture 2000 U.S. National Parasite Collection. <missing URL>
Uzmann, J. R.; H. J. Rayner (1958) Record of the parasitic copepod Lernaea cyprinacea L. in Oregon and Washington fishes., Journal of Parasitology 44(4): 452-453
Vaughan, Gene E.; Coble, Daniel W. (1975) Sublethal effects of three ectoparasites on fish, Journal of Fish Biology 7: 283-294
Wilson, Charles Branch (1918) Economic relations, anatomy, and life history of the genus Lernaea., Bulletin of the United States Bureau of Fisheries 35: 163-195